Biomedical Engineering Reference
In-Depth Information
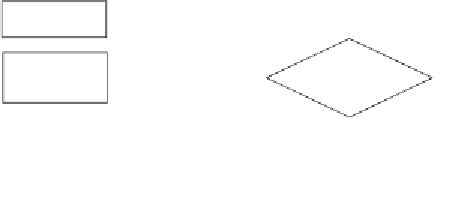
New
supplier?
N
Procurement
Y
Relevant
certificates?
N
Abort
Y
N
Audit
required?
Order
sample
Y
Audit plan
N
Conduct
audit
Satisfactory?
Y
Complete report, sign
agreements, and file
Figure 10.1
Example new supplier procedure.
10.3.1 Sterile Packaging
Sterile packs tend to come in two forms: flexible wrapped and rigid blister. The flexible wrapped
kind is the sort of packaging that you would see with any sterile wound dressing you would
purchase from a chemist (drug store). This type of pack is reserved for relatively light objects such
as adhesive dressings, small bone screws, and giving sets. The blister pack is for heavier objects:
ones whose shear bulk would damage the weaker wrappings of the former. For general use the
sterile packaging would come single wrapped, that is, there is only one seal between the device
and the outside world (
Figure 10.2
). If, however, the device is going into a sterile environment then
it would be double wrapped (
Figure 10.3
). The simple reason is that the inner pack will remain
sterile and can be passed to a sterile operative. If it were single packed the packaging itself would
be nonsterile and hence cannot be passed on to anyone in the sterile field. For this reason all
devices bound for the operating theater (OR) are, almost exclusively, double wrapped (
Table 10.2
).
There is a range of materials from which the pouches can be made, including:
l
63 gsm PeelPlus
l
1073B Tyvek
l
12/38 PET/PE
l
60 gsm paper
l
12 mu PET/9 mu foil/50 mu Peel PE



















Search WWH ::

Custom Search