Biomedical Engineering Reference
In-Depth Information
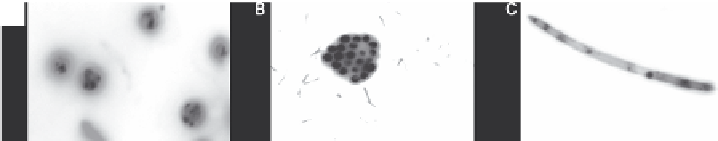
A
B
C
FIGURE 3.2 (See color insert.)
Nile Red stained
Chlorella
sp.: (a) unidentified chlorophyta,
(b) and
Navicula
sp., (c) viewed at 1000× using a Zeiss Axioskop epifluorescence microscope
at 490-nm excitation and 585-nm emission filter. Neutral lipid globules in the cytosol are
stained yellow. (Unpublished data.)
Govender et al., 2012). This facilitates the fluorescence distinction of lipid bodies,
resulting in better resolution and thus is important for seamless confocal imaging
(Cooper et al., 1999). Furthermore, unlike Nile Red, BODIPY 505/515 does not fix
to cytoplasmic constituents other than lipid bodies and chloroplasts. This discerning
property of BODIPY 505/515 to bind to lipid bodies alone offers rapid screening
and isolation of hyper-lipid producing algal strains. Bigelow et al. (2011) developed
a rapid, single-step, laboratory-scale in-situ protocol for GC-MS (gas chromatogra-
phy with mass spectroscopy) lipid analysis that requires only 250 µg dry mass per
sample. When coupled with fluorescent techniques using Nile Red or BODIPY dyes
and flow cytometry for cell sorting, the aforesaid GC-MS analysis allows throughput
screening of lipid-producing algal strains from varied environments. Upon isolation,
purification, and identification of a hyper-lipid producing algal strain, the researcher
would be interested in the physiological traits such as the photosynthetic efficiency,
carbon fixation rate, growth rate, etc. Alternatively, infrared analysis, which does not
depend on stain application but rather detects specific molecular absorption bands to
give approximate concentrations, can be used for the detection of many metabolites,
including lipids. This method has recently been applied to detecting changes in algal
cell composition during nitrogen starvation (Dean et al., 2010).
Spectroscopic methods such as near-infrared (NIR) and Fourier transform infra-
red (FTIR) spectroscopies have been established to predict the levels of spiked polar
and neutral lipids in algal cells based on multivariate calibration models (Laurens
and Wolfrum, 2011). The above infrared spectroscopic techniques are rapid, high-
throughput, and non-destructive means of algal screening for lipids. Hence, this cal-
ibration model serves as a short-time, high-throughput method of quantifying cell
lipids compared to time-consuming traditional wet chemical methods. The NIR and
FTIR spectra of biomass of various species accurately predicted the levels of lipids.
This fast, high-throughput spectroscopic lipid fingerprinting method is pragmatic in
real-time monitoring of lipid accumulation or a multitude of screening efforts that are
ongoing in the microalgal research community. Coherent anti-Stokes Raman scatter-
ing microscopy is also an associated technique that creates an image of whole cells
based on the vibrational spectra of a specific cellular constituent. Huang et al. (2010)
demonstrated that Stokes Raman spectroscopy could accomplish detection and identi-
fication of cellular storage lipids, specifically triglycerides. Further, similar to infrared
spectroscopic techniques, Raman scattering microscopy is also prospective as a rapid,
noninvasive compositional analysis method that enables imminent in-line or at-line

Search WWH ::

Custom Search