Biomedical Engineering Reference
In-Depth Information
(
A
)
(
B
)
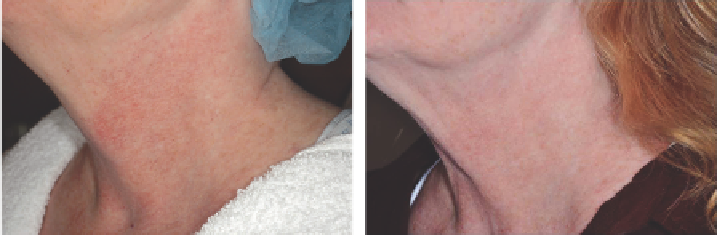
Figure 2.19
Poikiloderma (
A
) before and (
B
) after 2 treatments with intense pulsed light (30 J/cm
2
, 10 ms).
The nevus simplex is a specifi c subset of capillary malforma-
tion that goes by a number of common names making its
classifi cation somewhat confusing. In general, nevus simplex
may be referred to colloquially as a “salmon patch” (57).
When located on the forehead/eyelid or nape of the neck, the
terms “angel's kiss” or “stork bite” may be used, respectively.
Such lesions have a characteristic predilection for the midline,
mostly commonly affecting the nape of the neck and the cen-
tral face (glabella, eyelids, nose, and upper lip); the occiput and
lower back may also be affected (57).
The expected course of nevus simplex is to fade with matu-
rity, with most lesions typically resolving within 1 or 2 years of
age. Some lesions, particularly those located at the nape of the
neck, have been known to persist without further darkening or
thickening (58). Persistent glabellar and sacral lesions have also
been referred to as “medial telangiectatic nevus” and “butterfl y-
shaped mark,” respectively, and have further muddied the
nomenclature waters (55).
PWSs (“nevus fl ammeus”) are another subset of capillary
malformations that are almost always congenital, although
they may be acquired secondary to trauma and, thus, may
develop in adolescence or adulthood (59). Unlike nevus sim-
plex, PWSs persist throughout a patient's life. They may
occur anywhere on the body. Typically, they grow propor-
tionately with the child and may appear to lighten during the
fi rst three to six months of life. This is a physiologic change
most likely due to the decrease in blood HgB concentration
(typically 15-17 g/dL at birth to a nadir of 8-10 g/dL by age
three months) and should not be interpreted as a sign of clin-
ical resolution.
Videomicroscopy of PWS has demonstrated two patterns
of vascular abnormality: type 1 consists of tortuous, superfi -
cial, dilated capillary loops (blobs or globular structures);
type 2 consists of dilated ectatic vessels (rings) in the deeper
horizontal vascular plexus or a mixed pattern. Vessel depth
and number may correlate with anatomic location as one
study demonstrated that PWSs in the V3, neck, and trunk
locations were more likely to have a superfi cial type 1 pattern
and typically responded well to laser treatment; on the other
hand, lesions in V2 or distal extremity locations were more
likely to have a deeper type 2 pattern and did not respond as
well (60).
Although the exact molecular pathogenesis of capillary mal-
formations remains unknown in the majority of cases, it is
believed that localized defects in pathways controlling embryo-
genesis, vasculogenesis, and angiogenesis play key roles. Several
specifi c mutations have recently been identifi ed that have
shed some light on possible etiologies. Mutations in
RASA1
,
encoding p120-rasGTPase-activating protein (p120-rasGAP),
along the Ras/MAPkinase pathway, have recently been identi-
fi ed in patients with atypical capillary malformations with or
without concurrent arteriovenous malformations (AVMs) or
arteriovenous fi stulas; this protein appears to be crucial in con-
trolling proliferation, migration, and cell death in a number of
tissues, including vascular endothelium (61). Developmental
endothelial locus-1 (Del-1), an extracellular matrix protein
adhered to by human umbilical vein endothelial cells, is
another protein being investigated for its potential to induce
formation of a vascular plexus with increased number of
capillaries (62).
adverse medical effects
In addition to their abnormal cosmetic appearance, untreated
PWSs tend to follow a predictable pattern of progression char-
acterized by darkening in color (from pink-red to violaceous
or deep purple), gradual thickening and nodularity (with pos-
sible bleb formation), and possible soft tissue hypertrophy and
bone overgrowth (with subsequent deformation and func-
tional impairment that may require surgical intervention)
(63,64). One study found that two-thirds of patients develop
hypertrophy and nodularity by age 46 years, with a mean age
of 37 years for hypertrophy. Giant proliferative hemangiomas
may also arise in PWSs and can develop without any prior
history of trauma.
PWSs can also present with an infl ammatory component
consisting of scaling, excoriations, oozing, and crusting,
resembling an eczematous dermatitis. Treatment with topical
steroids to decrease the infl ammation can help, and the PDL
may be curative even after a single treatment (65,66).
The most serious extracutaneous fi ndings of PWS may
be glaucoma and the possible association with Sturge-Weber
syndrome (SWS). A retrospective case-control study from a
major ophthalmology referral center looked at 216 patients,
mean age 3.25 years, with unilateral or bilateral PWSs in the
ophthalmic (V1) and maxillary (V2) divisions of the trigemi-
nal nerve. The authors identifi ed the following risk factors for
glaucoma: bilateral PWSs (
P
= 0.0001), upper and lower eyelid
involvement (
P
< 0.0001), episcleral hemangioma (
P
< 0.0001),
iris heterochromia (
P
= 0.004), or choroidal hemangioma
(
P
< 0.0001) (67,68).
SWS is a sporadic congenital disorder characterized by the
classic triad of facial PWS invariably involving V1 (although it