Biology Reference
In-Depth Information
and are available for straightforward modification, cloning, and production.
Yeast production is fast, cheap, and can be scaled up using fermentation
technology. The advantages of yeast over
are that yeast cells are able
to secrete soluble proteins and perform post-translational modifications,
such as proteolytic processing, phosphorylation, and glycosylation.
E. coli
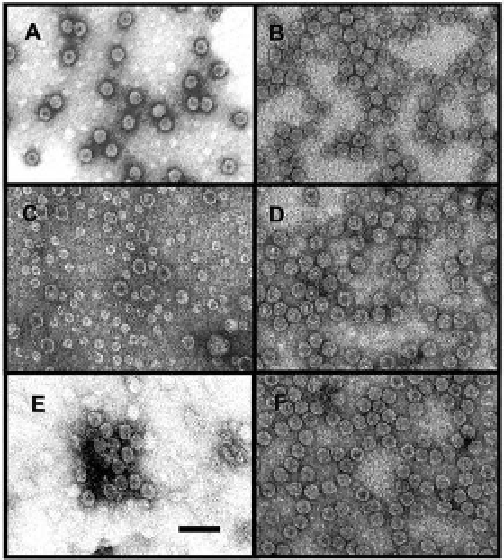
Figure 3.3
Transmission electron micrographs comparing
Cowpea chlorotic mottle
virus
expression system. (A) Wild-type CCMV containing RNA purified from cowpea plants
and (B) wild-type CCMV VLPs produced in the heterologous
(CCMV) purified from plants with CCMV VLPs purified from the
P. pastoris
P. pastoris
system. Mutants
∆
produced in the
34 = altered interior surface
charge (a range of particle sizes are observed) (C), SubE = deleted N-terminus (D),
CPPep11 = peptide insertion in surface-exposed loop (E), and 81/148 = alteration of
subunit interfaces at the metal binding sites (F). The scale bar is 100 nm. Reproduced
with permission from Brumfield, S., Willits, D., Tang , L., Johnson , J. E., Douglas, T., and,
Young, M. (2004) Heterologous expression of the modified coat protein of
P. pastoris
expression system included N
Cowpea
chlorotic mottle bromovirus
results in the assembly of protein cages with altered
architectures and function,
J. Gen. Virol.
,
85
(Pt 4), 1049-1053.
VLPs of the following viruses have been successfully produced in yeast:
the plant viruses BMV (
S. cerevisiae
) and CCMV (
P. pastoris
), as well as the
bacteriophage Q
b
(
S. cerevisiae
and
P. pastoris
) (Brumfield
et al.
, 2004;
Freivalds
, 1999). Intact VLPs are typically self-
assembled within yeast and can be isolated in high yields by cell lysis and
differential ultracentrifugation. Yields of up to 0.5 g VLP per liter cell culture
et al.
, 2006; Krol
et al.

Search WWH ::

Custom Search