Biology Reference
In-Depth Information
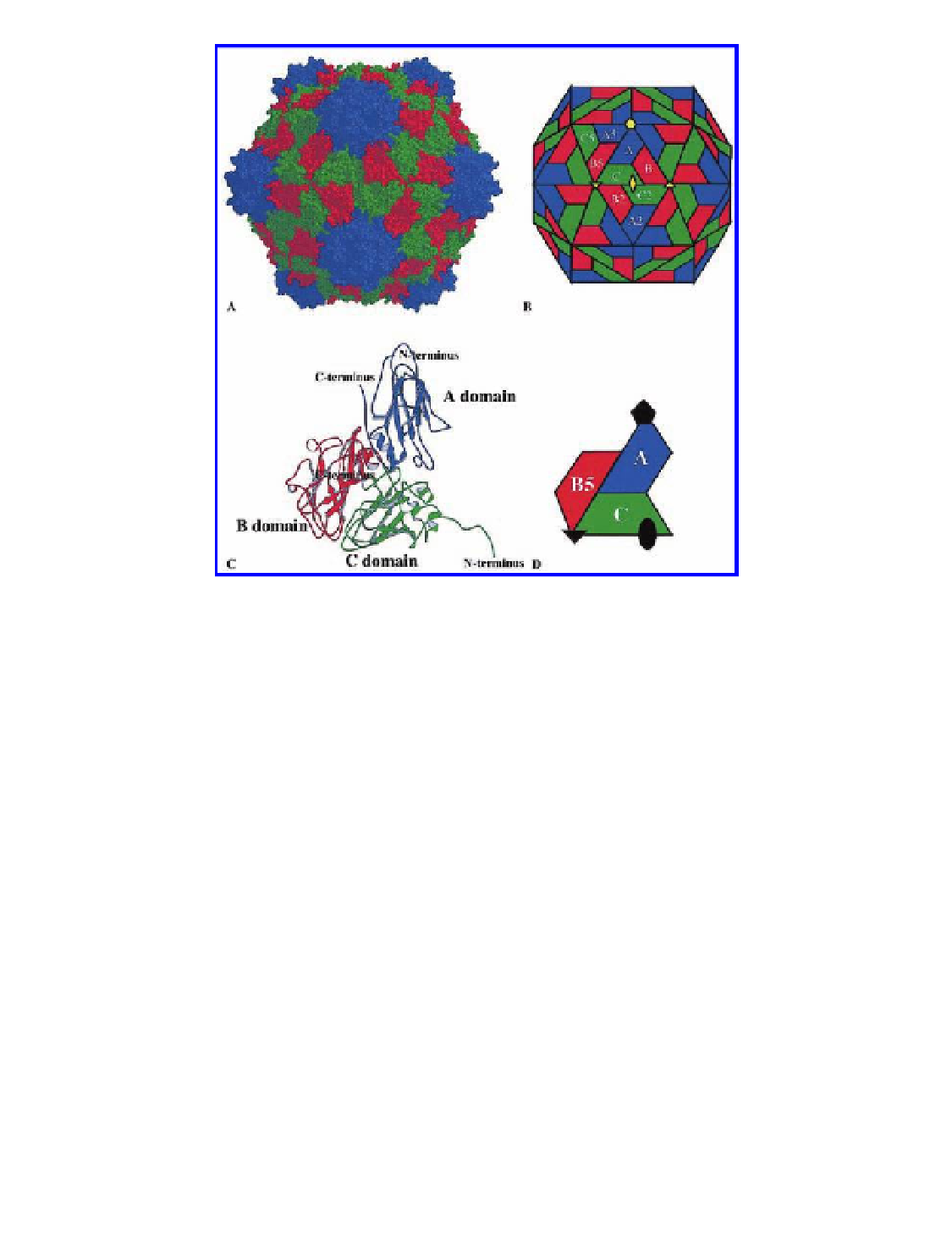
Figure 2.6
The structure of the viral capsid and the icosahedral asymmetric unit of
Cowpea mosaic virus
(CPMV). The capsid is comprised of two viral proteins, the S and
L subunits, which form three
b
-sandwich domains in the icosahedral asymmetric
unit. These three domains occupy comparable positions shown in a
= 3 quasi-
equivalent surface lattice. These are designated A (domains forming pentamers)
and B and C (domains forming hexamers). Numerals following the letter indicate
positions related by the icosahedral symmetry to A, B, and C. The S subunit forms
the domain occupying the A positions in an icosahedral lattice and these are shown
in blue. The L subunit is formed by two domains, C and B5, shown in green and red,
respectively. (A). A space-filling drawing of the CPMV capsid. All atoms are shown
as spheres of 1.8 Å in diameter. (B). A schematic presentation of the CPMV capsid.
The S subunit occupies A positions around the fivefold axis; the two domains of
L subunit occupy the B and C positions. The quaternary interactions at A/B5 and
C/C2 interfaces are pseudo-equivalent. Selected icosahedral symmetry axes are
also shown. (C). A ribbon diagram of the three
T
-barrel domains that comprise the
icosahedral asymmetric unit. N-termini of the S and L subunits are in the interior,
and C-termini are in the exterior. All three domains are variants of canonical jelly-
roll
b
b
sandwiches. (D). A schematic diagram of the asymmetric unit with icosahedral
symmetry axes. The A domains surround the fivefold axes, and the B and C domains
alternate around the threefold axis. Reproduced with permission from Lin, T.,
Chen, Z., Usha, R., Stauffacher, C. V., Dai, J. B., Schmidt, T., and Johnson, J. E. (1999)
The refined crystal structure of
Cowpea mosaic virus
at 2.8 Å resolution,
Virology
,
265
(1), 20-34.
Search WWH ::

Custom Search