Biology Reference
In-Depth Information
binding properties of CPV, which utilizes TfR in order to infect cells. Non-
infectious and non-replicating CPV VLPs were developed that preserve
binding to TfR (Singh
et al
., 2006a). Delivery of dye-labeled CPV VLPs to
different cancer cell lines
in vitro
has been demonstrated (Fig. 8.7) (Singh
et
al
., 2006a).
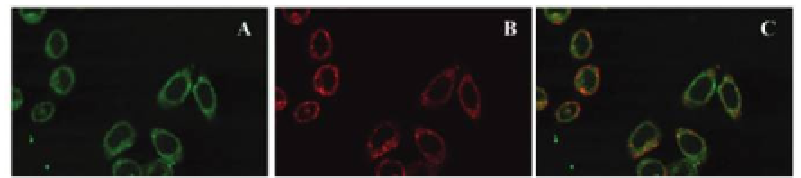
Figure 8.7
(CPV) VLPs into HeLa
cells (human cervical cancer cell line). HeLa cells incubated with Texas red-labeled
transferrin (red) and CPV VLPs were washed and fixed. Labeled antibodies (green)
were used to detect the presence of CPV VLPs in the cells by fluorescence confocal
microscopy. (a) CPV VLPs are seen as green areas in the cytoplasm, (b) localization
of Texas red-transferrin (red) and (c) merged picture showing co-localization of CPV-
VLPs and transferrin in yellow. Scale bar, 25 µm. Reproduced from Singh, P., Destito,
G., Schneemann, A., and Manchester, M. (2006) Canine parvovirus-like particles, a
novel nanomaterial for tumor targeting,
Binding and internalization of
Canine parvovirus
J. Nanobiotechnol.
, 4, 2.
Another receptor that is overexpressed specifically on tumor cells is
the receptor for folic acid (FA). FA is a vitamin required during growth
and development. Proliferating cells require high levels of this vitamin and
therefore overexpress the FA receptor (FR). Targeting nanomaterials to FR
has been shown to be a promising strategy (Cafolla
et al
., 2002; Chen
et al
.,
1998; Leamon & Low, 1992, 1993, 2002a,b; Reddy & Low, 1998; Turek
et al
.,
1993; Wang & Low, 1998). The VNPs CPMV and
Hibiscus chlorotic ringspot
virus
(HCRSV) have been covalently modified with FA. Multivalent display
of FA on the particle surface facilitated cell internalization by tumor cells
overexpressing FR (Destito
., 2007). In the case of CPMV,
particles were covalently modified with PEG and FA; PEG provides shielding
of the natural CPMV interactions, and FA facilitated target specificity (Fig.
8.8) (Destito
et al
., 2007; Ren
et al
., 2007). The FR-targeting strategy has been further
developed for targeted delivery of the chemotherapeutic doxorubicin using
the HCRSV platform (see Section 8.5) (Ren
et al
et al
., 2007).




Search WWH ::

Custom Search