Biology Reference
In-Depth Information
It has been suggested that surface vimentin is overexpressed in tumor
endothelium (van Beijnum
., 2006); this correlates with high uptake
of CPMV in tumor endothelium as shown in studies using the chick
choreoallantoic membrane tumor model. Fluorescent-labeled CPMV
sensors accumulated within tumor endothelium and allowed imaging and
mapping over long time periods (Fig. 8.6) (Lewis
et al
., 2006). The use of
CPMV as a natural endothelial probe is envisioned in imaging vascular
disease and may provide novel insights into the expression pattern of
surface vimentin.
et al
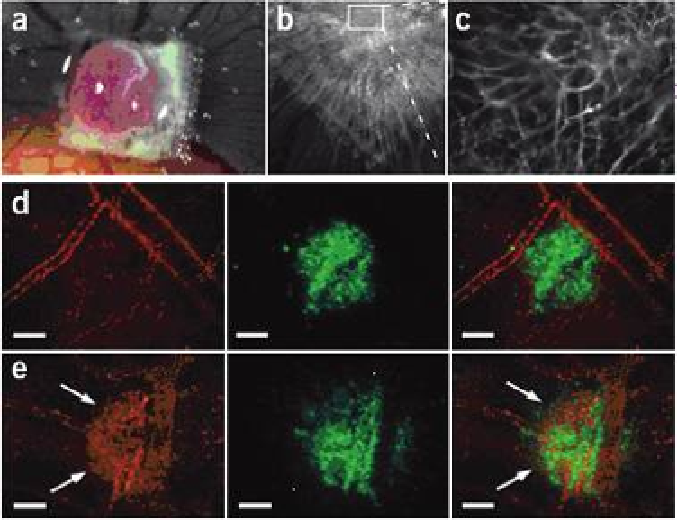
Figure 8.6
(CPMV) nanoparticles highlight tumor
angiogenesis: intravital imaging in a CAM/HT1080 fibrosarcoma model. (a) Bright-
field image of HT1080 tumor on-plant on the chick chorioallantoic membrane
(CAM) at 7 d (d = days). Opaque object is a nylon mesh grid used for quantification
of angiogenesis. (b) Fluorescence image of tumor on-plant after injection of
embryo CPMV-A555. (c) High-magnification image of tumor interior shown in (b);
tumor microvasculature is clearly visualized . (d, e) Visualization of HT1080 tumor
angiogenesis using CPMV-A555. (d) Left, visualization of pre-existing vasculature
in the CAM immediately after HT1080 tumor cell injection using CPMV-A555.
Middle, GFP-expressing HT1080 tumor bolus under the surface of the CAM. Right,
merge. Scale bar, 100 mm . (e) Left, visualization of pre-existing CAM vasculature
and neovasculature arising from tumor angiogenesis 24 h after tumor cell injection.
Middle, GFP-expressing HT1080 tumor bolus. The extensive migration over 24 h
indicates a high level of tumor cell viability. Right, merge. Scale bar, 100 mm.
Reproduced from Lewis, J. D., Destito, G., Zijlstra, A., Gonzalez, M. J., Quigley, J. P.,
Manchester, M., and Stuhlmann, H. (2006) Viral nanoparticles as tools for intravital
vascular imaging,
Fluorescent
Cowpea mosaic virus
Nat. Med.
,
12
(3), 354-360.

Search WWH ::

Custom Search