Biology Reference
In-Depth Information
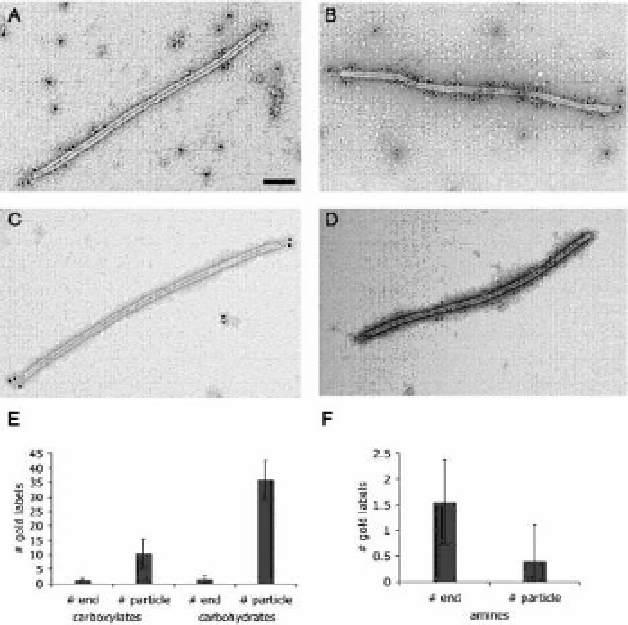
Figure 4.13
Immunogold staining of biotinylated SIRV2 particles using gold-labeled
anti-biotin antibodies and transmission electron microscopy. (A) Biotinylated
SIRV2 particles labeled using carboxylate-selective chemistry, (B) biotinylated
SIRV2 particles labeled at carbohydrates, (C) biotinylated SIRV2 particles labeled
using amine-selective bioconjugation techniques, and (D) non-modified SIRV2 used
as a control. (E, F) Ten particles each were further analyzed and the gold labels
per particle at the virus body and the virus end structures (tail fibers), respectively,
counted. Note difference in scale of (E) and (F). (F) Statistical analysis of differences
between virus body and end labeling was performed using Student's two-tailed
t
= 0.0078. Reproduced with permission from
Steinmetz, N. F., Bize, A., Findlay, K. C., Lomonossoff, G. P., Manchester M., Evans,
D. J., and Prangishvili, D. (2008) Site-specific and spatially controlled addressability
of a new viral nanobuilding block: sulfolobus islandicus rod-shaped virus 2,
Adv. Funct. Mater.
-test (Microsoft Excel) where
P
,
18
, 3478-3486.
structural proteins. Both the major and minor coat proteins are accessible for
modification using carboxylate- and carbohydrate-selective chemistries.
However, only the minor coat protein that forms the end structure can
be labeled using amine-selective chemistries (Fig. 4.13) (Steinmetz
et al.
,
2008a).
The ability to selectively attach labels and functionalities to the end
structures versus virus body opens the door for the development of highly

Search WWH ::

Custom Search