Biology Reference
In-Depth Information
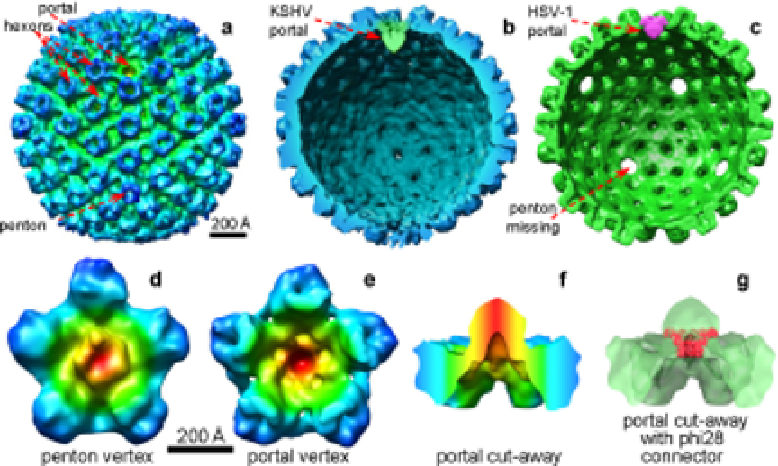
Fig. 5.
Identification of portal complex in KSHV and HSV-1. (
a
) Radially
colored surface representation of an averaged KSHV A-capsid cryoET recon-
struction, as viewed along a two-fold axis. A unique portal vertex devoid of
penton densities is identified at one of the 12 vertices. (
b
) Cut-away view of
(
a
) showing the portal in green. (
c
) Cut-away view of the cryoET structure
of chemically treated HSV-1 capsids.
69
Note: except for the portal vertex, all
other vertices are unoccupied, indicating that all pentons were removed by
the treatment. (
d
) One KSHV penton vertex with its five neighboring hex-
ons. (
e
) The KSHV portal vertex and its five neighboring hexons. Maps in
(
d
-
f
) are colored based on radial distance from the vertex axis. The densities
closest to the vertex axis are shown in red, with the color changing first to
green and then to blue to indicate densities that are further from the axis.
(
f
) Side view of (
e
) with upper portion removed for clarity. (
g
) Fitting of the
bacteriophage phi-29 portal connector complex at 10 Å (red) into the por-
tal of KSHV portal vertex (semitransparent green). (Adapated with permi-
sion from publishers and the authors.
69-70
)
remove the portal protein/complex along with the pentons. Notably,
the KSHV capsids were not subjected to any such chemical extraction,
so no ambiguities exist regarding the possible detachment of portal or
portal-associated proteins.
70
The portal structure presented by Chang
et al.
in the chemically treated HSV-1 capsid is essentially identical to