Biology Reference
In-Depth Information
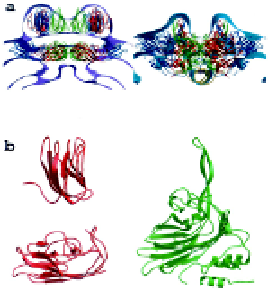
Fig. 2.
Cross-sectional views of the particle coat of MGNNV (
left
) and PaV
(
right
) defined by the cryoEM density.
(Reprinted from Tang
et al.
, 2002.)
domain that is similar to that in TBSV. Residues 217 to the C-terminal
residue 338 would then form a protruding domain that corresponds
to the surface protrusions observed in the cryoEM map.
The
-sandwich domain (residues 103 to 271) and the protrud-
ing domain (residues 272 to 387) of TBSV were manually fitted into
the inner shell and protruding density of the cryoEM map of
MGNNV, respectively. The resultant model was subjected to cycles of
rigid body refinement against a set of structure amplitudes generated
from the cryoEM map. This adjustment ensured that the refinement
was carried out against a molecular volume and shape. During the
refinement, each domain was defined as an independent rigid group.
The final model agreed well with the cryoEM map. Compared to the
original TBSV coordinates, the
β
-sandwich domain was shifted slightly
toward the center of the virus particle whereas the protruding domain
moved by 24 Å with a rotation of 87
β
. Such a large change reflects an
improved fit to the density but also suggests, like the 3D-PSSM
searches, that there may be no relationship between the folds of the
outer domains in TBSV and MGNNV and that the TBSV domain is
only shifted to optimally occupy the MGNNV density. Nevertheless,
the pattern of interactions between the outer domains confirms the
T
°
3 lattice of the capsid. These domains make more extensive con-
tacts at the icosahedral twofold axes than at the quasi-twofold axes,
which is consistent with the observation of more significant density at
=