Biology Reference
In-Depth Information
10
9
10
8
10
7
10
6
10
5
10
4
10
3
10
2
C. HIV1G/2F5/2G12
10
9
10
8
10
7
10
6
10
5
10
4
10
3
10
2
10
8
10
7
10
6
10
5
10
4
10
3
b12
A.
B. 2G12
048 2 6 0
0
4
8 12 16 20
Week
0 1020304050
Time (days)
60
Week
Parren
et al
. (2000);
J Virol
75: 8340
8347
Mascola
et al
. (2000);
Nature Med
6: 207
−
−
210
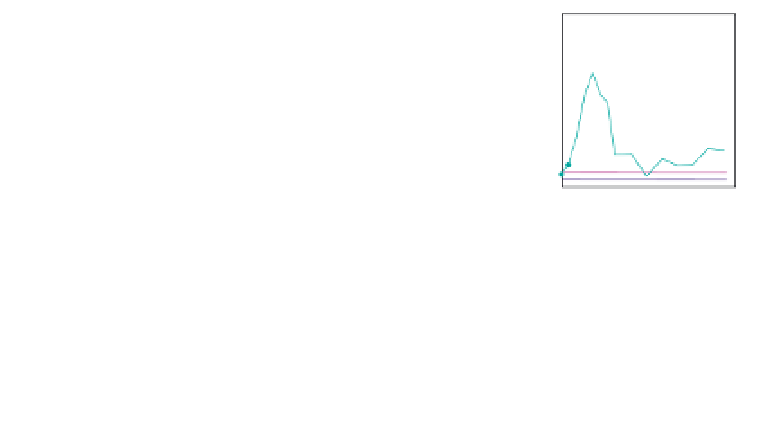
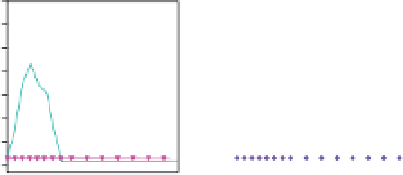
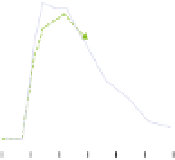
Fig. 6.
Dose-dependent protection conferred by either single (b12, and
2G12) or a combination of neutralizing antibodies (2F5, and 2G12) to ani-
mals in passive transfer experiments as demonstrated Refs. 188 and 189.
antibody
91
against a challenge infection by a TCLA isolate. Subse-
quently, Conley
et al
. demonstrated the efficacy of mAb 2F5 in par-
tially protecting chimpanzees against intravenous challenge with a
primary isolate.
92
The limited availability of non-human primates, par-
ticularly chimpanzees, led the investigators to develop an
in vivo
rodent model using severe combined immunodeficient (SCID) mice
transplanted with human PBL (hu-PBL-SCID mice) to study the pro-
tection afforded by mAbs against HIV-1 infection.
93,94
Using this
model it has been shown that passive administration of neutralizing
mAbs prior to or shortly after challenge could protect the mice
against a challenge infection.
95-97
In this model the antibody concen-
tration needed to protect against the challenge infection
in vivo
was
10 times higher than the concentration needed to neutralize the same
isolate
in vitro
.
98
It is interesting, but perhaps not surprising, to
observe the differences in the protective efficacy of these neutralizing
mAbs
in vivo
and
in vitro
. There are several factors that may influ-
ence the protective efficacy of these mAbs, such as dose of the challenge
virus and also the growth kinetics of the virus
in vivo
versus
in vitro
.
Parren
et al
. demonstrated that higher antibody concentration is
required to neutralize primary isolates compared to T-cell adapted
isolates.
99
Similar observations were made by Mosier and colleagues