Biology Reference
In-Depth Information
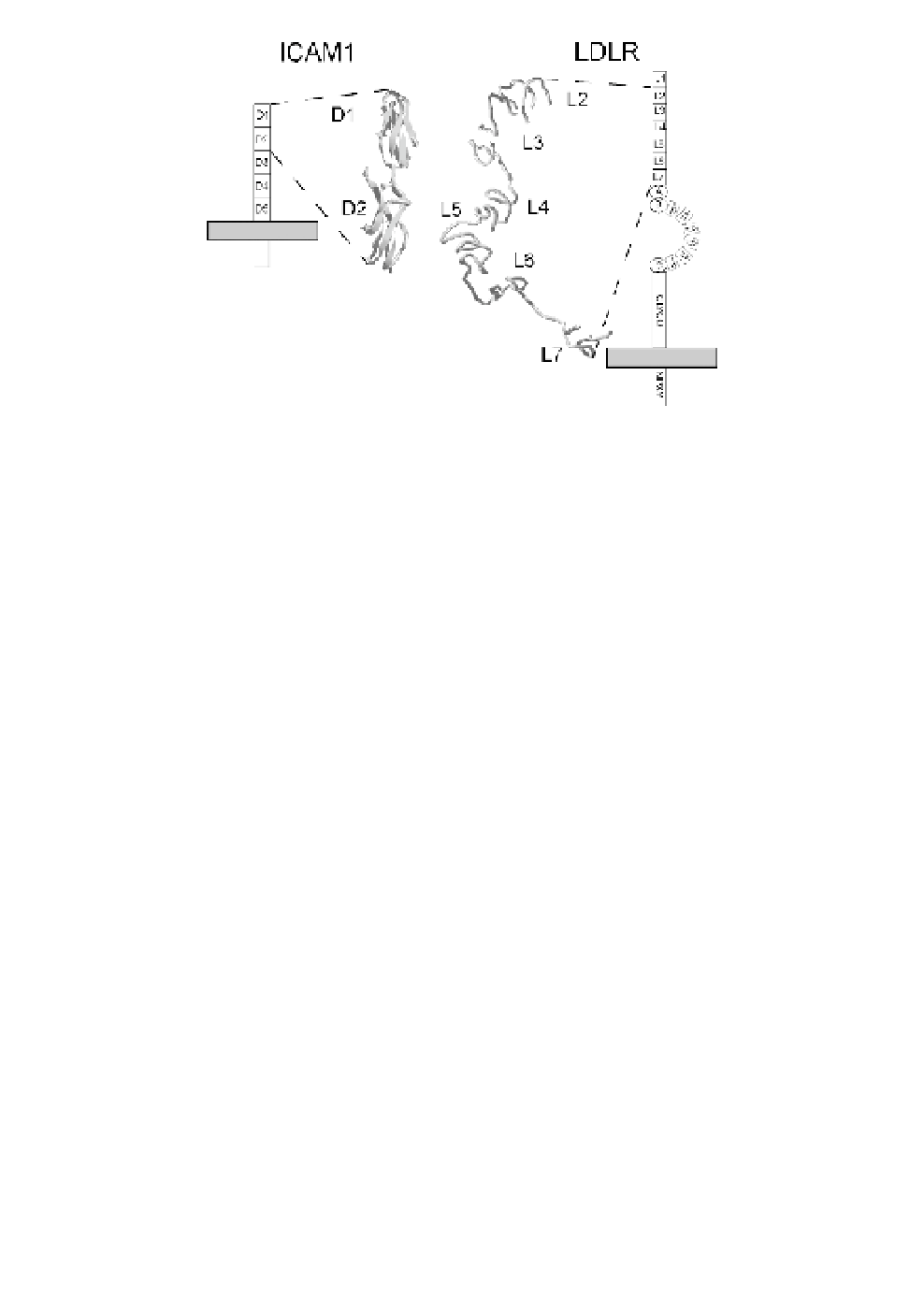
Fig. 1.
Comparison of the two receptors used by human rhinoviruses for
cell entry. The structure of the first two immunoglobulin domains of the
major group receptor ICAM-1
43
and of six ligand binding repeats (L2
through L7; L1 is disordered in the structure and not seen) of LDLR at
pH 5.3
44
are shown. The drawings schematize the orientation of the recep-
tors with respect to the plasma membrane (grey rectangle) and include
other domains present in the native receptors. A, B, and C denote domains
with similarity to epidermal growth factor precursor. Empty rectangles sym-
bolize the YWTD domains forming a
-propeller. Note the absence of a
coated pit localization signal (NPXY) in ICAM-1.
β
different serotypes since the geometry of the cleft would prevent
entry of antibodies and thus immunological pressure; at the same
time, the cleft would be accessible for a slim receptor molecule.
35
This proved to be true in part since the receptor used by the major
group of HRVs, the slender ICAM-1 molecule (Fig. 1), indeed
binds within the canyon
36
and the residues in this region appear to
be somewhat more conserved than the loop residues.
37
Alignment
of VP1 sequences of all major group HRVs with respect to non-con-
tiguous amino acid residues known to contact ICAM-1 in HRV3,
14 and 16, revealed two patterns strictly conserved within
37a
the two
subgenera. However, antibodies can also penetrate rather deeply
into this cleft.
38
The canyon hypothesis was further challenged by the discovery
that, in the minor group HRVs, the receptors do not bind within the