Geoscience Reference
In-Depth Information
1.0
0.8
CO
2
HCO
−
CO
2−
0.6
1.0
0.8
0.4
0.6
0.4
0.2
0.2
0.0
2468 0 2
0.0
7.0
7.5
8.0
8.5
pH
T
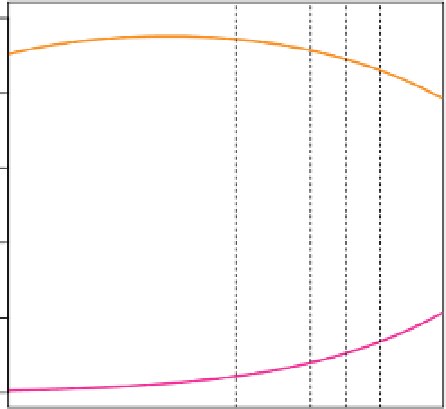
Figure B1.1
Bjerrum plot showing the relative contributions of carbon dioxide (CO
2
), bicarbonate (HCO
3
-
), and carbonate (CO
3
2-
) to the dissolved
inorganic carbon as a function of pH
T
at 15°C and a salinity of 35. K
1
and K
2
were calculated according to Lueker et al. ( 2000 ). The dashed vertical
lines indicate the average open-ocean surface pH
T
during the Last Glacial Maximum (LGM), 1766, 2007, and 2100 (see Table 1.1). The i gure is drawn
with the Bjerrum function 'seacarb' ( Lavigne and Gattuso, 2010 ).
pH
pH is a measure of ocean acidity (pH = -log
10
[H
+
]) which can
be reported using different scales: National Bureau of Standards
(pH
NBS
), seawater (pH
SWS
), free (pH
F
), and total (pH
T
) scales. The
pH values on the NBS and SWS scales are, respectively, about
0.15 units higher and about 0.01 units lower than on the total
scale. This makes data compilation and analysis difi cult because
some conversions from one scale to another are not
straightforward. The total scale is recommended (Dickson 2010)
and is the scale used in this topic whenever possible.
magnesium whereas it is higher at high (>12%) mole
fractions (Dickson 2010). The dissolution equilibrium is:
2
+
2
−
CaCO
=
Ca
(aq)
+
CO
(aq)
(B1.6)
3
3
with the equilibrium constant dei ned as the solubility
product for calcite or aragonite:
*
2
+
2
−
K
=
[Ca
]
[CO
]
.
(B1.7)
sp
sat
3
sat
The CaCO
3
saturation state is dei ned as the ratio between
the observed ion product and the expected ion product
when the solution is in equilibrium with a particular
calcium carbonate mineral:
Calcium carbonate
Three major biogenic calcium carbonate (CaCO
3
) minerals
occur in seawater: aragonite, calcite, and magnesian calcite
(Mg-calcite). Aragonite is about 1.5 times more soluble
than calcite. Mg-calcite is a variety of calcite with
magnesium ions substituted for calcium ions. Its solubility is
lower than that of calcite at low (< 4%) mole fractions of
2
+
2
3
−
s
*
[Ca
][CO
] /
K
.
(B1.8)
W
=
Seawater is in equilibrium with that mineral when Ω =1,
supersaturated when Ω > 1 (which promotes inorganic
precipitation), and is undersaturated when Ω < 1 (which
promotes inorganic dissolution).
The increase in surface-ocean dissolved CO
2
is
proportional to that in the atmosphere (upon equili-
bration after about 1 year) but the increase in
C
T
is not. This is a result of the buffer capacity of sea-
water. The relative change of dissolved CO
2
to the
relative change of
C
T
in seawater in equilibrium
with atmospheric CO
2
is described by the so-called
Revelle factor according to which a doubling in
atmospheric CO
2
only leads to an increase in
C
T
of
about 10% (Zeebe and Wolf-Gladrow 2001; see also