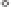Geoscience Reference
In-Depth Information
Atmosphere
cloud
formation
sun
new particle/
CCN formation
albedo
O
2
ozone
destruction
I, IO,
Br, BrO
transfer of I to
terrestrial ecosystems
in rain and aerosols
O
3
photolysis
and oxidation
CH
3
I, CHBr
3
seaweeds
Land
DOM/POM
Ocean
bacteria
phytoplankton
Figure 11.2
Pathways of organohalogen formation in the surface ocean, and the atmospheric and climatic consequences of the sea-to-air l ux. DOM,
dissolved organic matter; POM, particulate organic matter.
the surface oceans that lead to a net l ux of these
gases to the atmosphere (see Fig. 11.2).
The production of organohalogens has been con-
i rmed in a wide range of marine organisms, includ-
ing phytoplankton (e.g. Tokarczyk and Moore 1994;
Moore
et al.
1996; Manley and Cuesta 1997), mac-
roalgae (Manley and Dastoor 1987; Nightingale
et al.
1995 ), and bacteria ( Amachi
et al.
2001 ). As a
result, high-productivity regions of the oceans, such
as upwellings and coastal areas, are considered to
be strong source regions of volatile organohalogens.
Biological production by macroalgae and phyto-
plankton occurs via two mechanisms: (1) methyla-
tion of organic halogens (common haloform
reaction) to produce monohalogenated compounds
(CH
3
X, X = Br, Cl, I; Urhahn and Ballschmiter 1998)
or (2) halogenation of organic precursors, catalysed
by haloperoxidases (BrPO and IPO) produced in
algal cells (Nightingale
et al.
1995 ; Manley 2002 ).
Production by macroalgae appears to be a response
to, or by-product of, photo-oxidative or mechanical
stress (Nightingale
et al.
1995 ; Manley 2002 ). In phy-
toplankton, production of organohalogens may
serve as a method of cellular halide excretion, pro-
tect against photo-oxidative stress, chemically deter
grazers, or simply represent a by-product of normal
metabolism (Manley 2002). Recent work has also
implicated marine bacteria as potential globally
important producers of organohalogens, with a
wide range of bacteria displaying methylating capa-
bilities (Amachi
et al.
2001 ).
Photochemical production of organohalogens
involves a reaction between photochemically pro-
duced methyl radicals and halogen atoms (Moore and
Zai riou 1994 ; Richter and Wallace 2004 ). As the
methyl radicals are likely to be derived from a biologi-
cal source, an indirect biogenic control on the process
exists (Richter and Wallace 2004). Dihalogenated com-
pounds can also be formed through the photochemi-
cal degradation of other organohalogens. For example,
CH
2
I
2
is photolysed to produce CH
2
ClI, with a yield of
25 to 30% (Martino
et al.
2005 ). A further, recently
described, source of organohalogens in seawater
involves ozone-mediated reactions between dissolved