Geoscience Reference
In-Depth Information
with organisms both driving and responding to the
geochemical conditions within the sediment.
Therefore, in order to understand the potential
impact of ocean acidii cation on sediment fauna
and the processes they support, it is useful to i rst
consider the key processes that control the distribu-
tion of CO
2
and pH in sediments.
The amount of CO
2
and the subsequent value for
pH can vary enormously between different sedi-
ments—to such an extent that when looking across
a range of marine environments, including temper-
ate and tropical coastal seas and deep-sea sedi-
ments, pH in the uppermost few centimetres of
sediment ranges from 6.5 to 8.2 (Aller and Yingst
1978 ; Martens
et al.
1978 ; Wenzhöfer
et al.
2001 ;
Hulth
et al.
2002 ; Zhu
et al.
2006b ; Burdige
et al.
2008). The reader should be aware that pH in these
studies is measured on different pH scales which
may differ by up to 0.17 units depending on tem-
perature (over the range 10-30°C) and salinity (over
the range 10-30). As temperature and salinity are
not always specii ed in the literature, we refer to the
relevant pH scales where examples of sediment pH
values are given in this chapter. CO
2
partial pres-
sure (
p
CO
2
) across a range of coastal and deep-
sea sediments was found to be between 0.4 and
16 000 μatm (Wenzhöfer
et al.
2001 ; Zhu
et al.
2006a ).
However, organic matter mineralization, when cou-
pled with relatively slow ventilation, typically leads
to a build-up of CO
2
and reduced metals and nitro-
gen, with a reduction in sediment pH. Consequently,
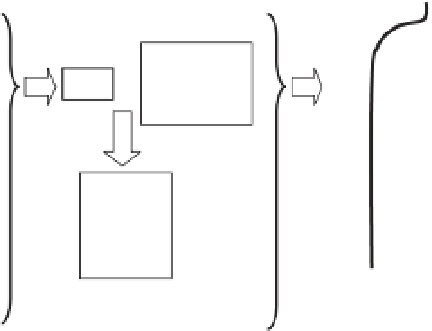
pH proi les down through the sediment (Fig. 9.1)
typically follow a sharp decrease from the top sedi-
ment layer to a pH minimum situated just below
the oxic zone (usually the top few millimetres or
centimetres) and subsequently pH becomes invari-
able with depth (e.g. Burdige
et al.
2008 ). Figure 9.2
shows that even under situations of extreme acidii -
cation in the overlying seawater, values for porewa-
ter pH
NBS
become more similar to those values
observed under control conditions as one moves
deeper into the sediment. These depth proi les are
driven by microbial mineralization of organic mat-
ter and involve a number of redox reactions which
follow in the order of decreasing Gibbs free energy
(ΔG
o
). For each redox couple (e.g. NO
3
-
-N
2
), the elec-
tron acceptor (in this case NO
3
-
) must be depleted
before the next most energetically favourable reac-
tion becomes dominant. Thereby, oxygen (O
2
) is
used i rst during aerobic respiration, followed by
nitrate (NO
3
-
), manganese (Mn), iron (Fe), sulphate
(SO
4
2-
) and i nally CO
2
or acetate during methano-
genesis. The relevant idealized redox reactions are
listed below in order of decreasing ΔG
o
:
CH O
+®
O
CO
+
H O (aerobic respiration)
2
2
2
2
Organic matter
(CH
2
O)
pH
6
78
Respiration (O
2
)
Denitrification
Oxic zone
Carbonate
minerals
CaCO
3
H
+
+
Mn reduction
Fe reduction
SO
2-
reduction
Methanogenesis
Ca
2+
HCO
-
CO
2-
CO
2
Figure 9.1
A summary of the diagenetic processes controlling pH distribution in sediments.