Geoscience Reference
In-Depth Information
0
50
P
50
P
90
ambient
P
O
2
100
150
P
50
(
Illex illecebrosus
)
200
250
300
0
2
4
6
8
10
12
14
P
O
2
(kPa)
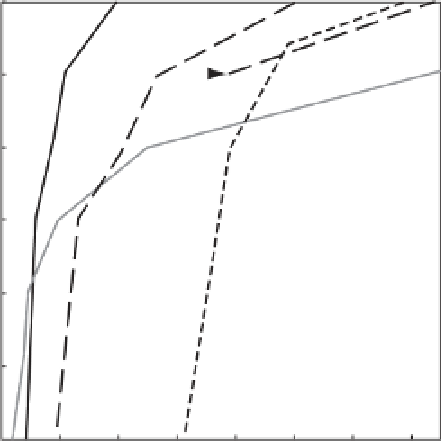
Figure 8.3
Relationship between blood-oxygen saturation and depth in the Humboldt squid, Dosidicus gigas (B. Seibel, unpublished data). The ambient
oxygen content of water in the Gulf of California decreases as a function of depth, resulting in reduced oxygen saturation of the blood. The P O
2
resulting in
50 and 90% saturation in D. gigas ( P
50
and P
90
) decreases with depth (i.e. the afi nity of haemocyanin for oxygen increases) due to reduced temperature at
depth. For comparison, the P
50
for another ommastrephid squid, Illex illecebrosus, is shown from the more oxygenated Atlantic ocean (dashed line; data from
Pörtner 1990 ). The lower P
50
of D. gigas rel ects an adaptation to the pronounced oxygen minimum zone in the eastern Pacii c. At depths above ~200 m, the
blood of D. gigas is at least 50% saturated. At depths shallower than ~130 m, its blood is at least 90% saturated. In contrast, I. illecebrosus would have to
stay at depths shallower than 100 m to achieve 50% saturation if it lived in the eastern Pacii c. The critical oxygen partial pressure (P
crit
) occurs near 160 m
for D. gigas. These numbers will change signii cantly with exposure to elevated CO
2
levels and uncompensated changes in blood pH as illustrated in shallow
water for D. gigas (see arrow). A P co
2
of 2000 μatm results in a 0.15 pH unit decrease in arterial pH assuming a buffering capacity similar to I. illecebrosus
(Pörtner 1990). The Bohr coefi cient of the D. gigas blood (∆logP
50
/∆pH = -1.1) results in a decrease in oxygen afi nity (P
90
at P co
2
= 2000 μatm, dashed
line). This effect may narrow the width of habitable water layers.
and then unloading of the pigment on each cycle
through the body, leaving no venous oxygen reserve.
Maximum oxygen transport in the blood, supple-
mented by oxygen provision via the skin of the
working mantle musculature, rel ects the optimized
capacity and maximum use of the oxygen supply
machinery in some swimming squids (Pörtner 1990,
1994). Consequently, muscular squids are thought to
live chronically on the edge of oxygen limitation
(Pörtner 2002) and are not well poised to adapt to
future environmental changes that inl uence oxygen
supply and demand (Fig. 8.3). Maintenance of extra-
cellular pH is particularly important, and any low-
ering of pH in arterial blood endangers the uptake
of oxygen from the water and its binding to haemo-
cyanin. This may occur in hypoxic, CO
2
-rich seawa-
ter as in oxygen minimum zones (OMZs).
Given such constraints, it is surprising that a large
ommastrephid squid, the Humboldt squid
Dosidicus
gigas
, is associated with the distribution of pronounced
OMZs in tropical oceans. These develop where the
water is thermally stratii ed and a high surface
productivity supports formation of a high biomass of
respiring organisms at depth. Nearly 8% of the
world's oceans contain less than 20 μmol O
2
kg
-1
(Paulmier and Ruiz-Pino 2009). Since oxygen con-
sumption is accompanied by CO
2
production, OMZs
also tend to have low pH and to be undersaturated