Geoscience Reference
In-Depth Information
200
150
S. pistillata
(Gattuso
et al
. 1998)
S. pistillata
(Reynaud
et al
. 2003)
G. fascicularis
(Marubini
et al
. 2003)
G. fascicularis
(Marshall and Clode 2002)
P. compressa
(Marubini
et al
. 2001)
P. compressa
(Langdon and Atkinson 2005)
P. lutea
(Ohde and Hossain 2004)
P. lutea
(Hossain and Ohde 2005)
P. compressa
(Marubini
et al
. 2003)
T. reniformis
(Marubini
et al
. 2003)
A. verweyi
(Marubini
et al
. 2003)
A. cervicornis
(Renegar and Riegl 2005)
M. mirabilis
(Horst and Edmunds unpubl.)
P. rus
(Horst and Edmunds unpubl.)
A. eurystoma
(Schneider and Erez 2006)
Fungia
sp. (Hossain and Ohde 2005)
1st order model
Regression of pooled data
100
50
0
0
1
2
3 4
Aragonite saturation state
5
6
7
8
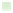
Figure 7.2
Relative rate of calcii cation for a number of coral species as a function of seawater aragonite saturation state (Ω
a
; from C. Langdon, pers.
comm.). References can be found in Appendix 7.1 available online at
http://ukcatalogue.oup.com/product/9780199591091.do
Nonetheless, this comes at a substantial energetic
cost, energy that would otherwise have been spent
on other essential processes such as protein syn-
thesis, growth, and/or reproduction, and this up-
regulation is probably not sustainable in the long
run. However, if organisms have sufi cient energy
resources in terms of food, nutrients, and light (for
those organisms dependent on photosynthesis),
they may be able to compensate for the additional
energy demand required to calcify under condi-
tions of elevated CO
2
. Overall, despite different
and even opposing responses between different
marine calcii ers and different responses between
the same species in different experiments, the
majority of experimental results to date strongly
suggest that ocean acidii cation will result in a
slowdown in calcii cation by benthic organisms.
Observations of linear extension and/or calcii ca-
tion rates in coral colonies from the Atlantic,
Pacii c, and Indian Oceans, including
Porites
colo-
nies on the Great Barrier Reef (Cooper
et al
. 2008 ;
De'ath
et al
. 2009) and in Phuket, Thailand (Tanzil
et al
. 2009 ),
Pocillopora
colonies in Panama
( Manzello 2010 ), and
Diploria
colonies in Bermuda
(A. Cohen, pers. comm.), show decreasing trends
in their rates for the past several decades. However,
it is not possible to conclude unequivocally that
this is a result of ocean acidii cation. A recent study
in the central Red Sea demonstrated a 30% reduc-
tion in the growth of
Diploastrea heliopora
since
1998, which was directly related to rising sea-sur-
face temperature (Cantin
et al
. 2010 ).
A small fraction of benthic CaCO
3
production is
chemically precipitated CaCO
3
, often as a direct
consequence of biological processes changing the
seawater chemistry and resulting in precipitation,
or dissolution and re-precipitation of a carbonate
mineral phase. Such carbonate cements serve an
important role in consolidating reef structures.
Ocean acidii cation could result in slower rates of
abiotic carbonate precipitation, changes in the aver-
age mineral composition of the cements, and weaker
carbonate reefs and structures (Andersson
et al
.
2005 ). Manzello
et al
. ( 2008 ) observed that cements
in intraskeletal pores were almost absent on coral
reefs in the eastern tropical Pacii c. These reefs expe-
rience high CO
2
and low Ω as a result of upwelling
and are, in general, poorly developed and subject to