Biomedical Engineering Reference
In-Depth Information
Chapter 1
Chitosan and Chitosan derivatives as Chelating
agents
Hiba M. Zalloum and Mohammad S. Mubarak
iNtroduCtioN
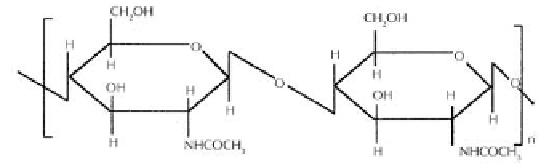
Chitosan, as shown in Figure 1, is a well-known hetero biopolymer (natural polysac-
charide) made of glucosamine and a fraction of acetylglucosamine residues (Krish-
napriya and Kandaswamy, 2010). It is a biodegradable, biocompatible, and nontoxic
natural polymer with a metal uptake capacity, (Jung et al., 1999; Rabea et al., 2003;
Sashiwa et al., 2003; Tsigos et al., 2000; Varma et al., 2004; Xing et al., 2005) that
can be obtained from the alkaline deacetylation process of the second most abundant
biopolymer, chitin as given in Figure 2, which is found widely in nature and can be
extracted from fungi, lobster, shells of shrimp and crab, and in the cuticles of in-
sects. (Brugnerotto et al., 2001a, 2001b; Heux et al., 2000; Kittur et al., 1991; Volesky,
2001). The main characteristics of chitosan are hydrophilicity, harmlessness for liv-
ing things and biodegradability, easy chemical derivatization, and capability to adsorb
a number of metal ions. Therefore, chitosan seems to be a very interesting starting
material for chelating resins (Katarina et al., 2006). The degree of polymerization
and deacetylation and the distribution of acetyl groups along the polymer chain are
of crucial importance for chitosan metal interacting characteristics. Making chemical
derivatives is a way to alter the metal interacting characteristics of chitosan (Onsoyen
and Skaugrud, 1990).
Figure 1.
Structure of Chitosan.
Figure 2.
Structure of Chitin.



Search WWH ::

Custom Search