Information Technology Reference
In-Depth Information
θ
θ
(a)
(b)
Figure
16.4.
Contact angle (y) between the liquid droplet and surface. (a) Contact
angle in the absence of electrical filed. (b) Contact angle in the presence of
applied electrical field.
property of electrowetting. Inspired by Lippmann's work [10], Kim's group
incorporates electrostatic charge to modify the capillary force at the liquid and
solid interface. The electrostatic force is directly related to the liquid surface
tension. When a liquid droplet is placed on a dielectric layer (Fig. 16.4), various
contact angles develop due to different hydrophobicities of the surface. The angle
represents a quantitative measurement of the wetting ability of a liquid to the
surface. When an electric field is generated between the liquid droplet and an
electrode underneath the dielectric layer, the contact angle is altered, giving rise to
a phenomenon known as electrowetting.
Based on Lippmann's equation, the tension
g
SL
in the interface between the
dielectric layer and liquid is defined as
CV
2
2
g
SL
ð
V
Þ¼g
SL
j
V
¼
0
;
ð
16
:
3
Þ
where V represents the electrical potential difference between liquid and the
electrode layer, and C denotes the specific capacitance of the dielectric layer.
By controlling the interface tension, E. S. Kim's group has developed digital
microfluidic device containing a glass cover (top) and patterned electrode under-
neath (Fig. 16.5). An asymmetric electrostatic field is generated to create
differential wettabilty between the front and rear sides of the droplet, thereby
developing pressure difference between the front and the rear to drive the droplet
to migrate forward. This driving mechanism is characterized as an electrowetting-
on-dielectric (EWOD) actuation.
V
1
V
2
<V
1
Figure
16.5.
Asymmetric pressure develops due to the asymmetric electrical field.
The droplet migrates to the left when V
2
is less than V
1
.








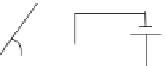









Search WWH ::

Custom Search