Information Technology Reference
In-Depth Information
Such view would lead to the definition of two Iron (II) metal ions surrounded by
four deprotonated, negatively charged carboxylic groups of the interconnecting
ligands. The formation of electro-neutral 4+/4-units will depend critically on the
smoothness of the deprotonation reaction of the carboxylic acid groups.
Alternative to the spontaneous deprotonation of carboxylic acid groups on
copper surfaces, a redox reaction involving the reduction of four carboxylic
protons under simultaneous oxidation of the two Iron(0) centers might be
considered. The formed gaseous hydrogen could easily migrate into the UHV
environment so favoring the accomplishment of the redox reaction. Further-
more, it has been mentioned that the principle of maximal occupancy of
the coordination sites might not be strictly valid under 2D-UHV confinement,
since the ligands tend to layer down onto the surface and additional solvent
molecules, present in conventional reaction conditions, are not present to fill
open coordination sites at the metal ion.
On the ligand side, it was shown that the lengths of linear rod-like ligands can
be two, three, or four phenyl units without losing their self-assembly ability. Thus,
the distance between the positions of two Fe-dimers within the metal ion network
can be deliberately chosen between 1.2 and 2.0 nm. Near-edge, X-ray adsorption
fine structure studies have shown that the aromatic backbones of the ligands are
adsorbed with their phenyl rings almost parallel to the Cu(1 0 0) surface plane [28].
Besides changing the lengths, the introduction of photoactive double bond
structures into the ligand backbone is possible. Interestingly, the introduction 2D
prochirality on the ligand side leads to less-ordered metal ion network structures,
1.5
3.5 nm
1.0
0.5
0.0
0
10
20 30
Position (A)
40 50
60
70
(a)
(b)
Figure
12.11.
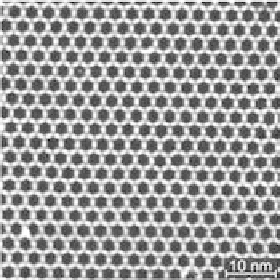
(a) The high resolution STM image represents the 2D topography
and the internal structure of the extended hexagonal Co-metal ion dicarbonitrile
terphenyl network indicating three-fold carbonitrile coordination around
monomeric Co-centers at the crossing points. Internal cavities of 3.5 A diameter
are generated on the Ag(111) surface. (b) STM image showing the extended
regular metal ion network topology [31].








Search WWH ::

Custom Search