Information Technology Reference
In-Depth Information
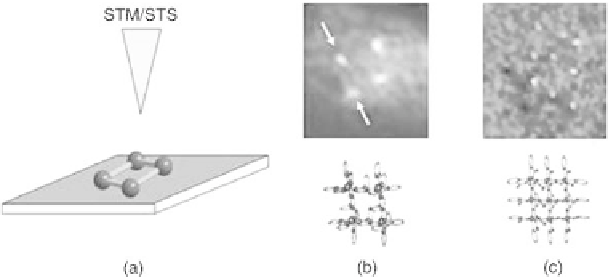
be gained by a scanning tunneling spectroscopy technique called current induced
tunneling spectroscopy (CITS) [26]. The experiments allowed the localization of
the positions of the incorporated Co
II
ions by a selective mapping of the highest
occupied molecular orbitals (HOMOs). Principle density functional theory
calculations confirmed that in this type of molecules the HOMOs possess a large
d-character, such that they are strongly localized around the positions of the metal
ions. Consequently, the projection (of the CITS maps at certain negative tunneling
biases) reveals electronically the cornerstone positions of the four Co
II
metal ions
(Fig. 12.8) [10]. The same technique was successfully applied to the higher
homologous [3
3] Mn
9
II
and [4
4] Mn
16
II
MIAs aligning respectively 9 and
16 manganese metal ions. The obtained CITS maps mirror the structural situation
within the metal ion arrays; although very regularly arranged, the metal ions
display in these higher homologues a more lozenge-like structure (Fig. 12.8). This
structural deviation from the optimal square-like arrangement can be attributed to
the ''pinching-in'' of the organic ligands during metal ion coordination, reflecting
the importance and the consequences of sufficiently instructed metal-ligand
interactions for the outcome the self-assembly processes [25].
In conclusion, the formation of highly ordered 2D monolayers of metal ion
arrays on surfaces represents a two-tiered self-assembly process: (i) The [n
n]
metal ion arrays are formed in a bulk self-assembly step in solution from their
molecular components (organic ligands and metal ions). Subsequently, (ii) the
[n
n] metal ion arrays are self-assembled themselves into densely packed
domains of monolayers on the graphite surface. The first self-assembly process
relies on the read-out of the coordination instructions stored in the ligands and the
metal ions, while the second is steered by van der Waals forces between the
metal ion arrays on one side and between arrays and graphite surface on the other
side. Due to the flat, square-like geometry of the metal ion arrays, this second
Figure
12.8.
(a) Schematic principle showing the metal ion array on a graphite
surface. (b) and (c) Show the results of the locally resolved current-induced
tunneling spectroscopy (CITS) measurements of a [22] Co
4
II
and [33] Mn
9
II
indicating the position and arrangement of the respective metal ions [26].






Search WWH ::

Custom Search