Information Technology Reference
In-Depth Information
d (Fe
II
−
N) = 2.15 Å
4 Fe
II
(HS)
1.00
300 K
p
LS(Fe
II
)
HS(Fe
II
)
T
h
ν
0.99
1.00
4.2 K
0.90
−
3
0
3
d (Fe
II
−
N) = 2.05 Å
(a)
4 Fe
II
(LS)
V/mms
−
1
(b)
(c)
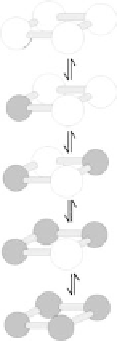
Figure
12.6.
Spin transition scheme of the [2 2] Fe
4
II
metal ion arrays exhibiting
switching between the diamagnetic low spin (LS) and the paramagnetic high
spin state. (a) and (b) The spin state change can be triggered by temperature,
pressure and light; the averaged Fe-N bond lengths in the 3 HS/1 LS and in the
1 HS/3 LS states are given. (c) The Moessbauer spectra at two different tempera-
tures showing the spin transition between the two Fe(II) species [20].
In addition, the magnetic anisotropy in a series of [2
2] Co
4
II
metal ion
arrays was investigated by single-crystal magnetization measurements at low
temperatures. The magnetization data exhibit metamagnetic-like behavior and are
explained by the weak-exchange limit of a minimal-spin Hamiltonian including
Heisenberg exchange, easy-axis ligand fields, and Zeeman terms [21].
12.2.2. Surface Organization of Metal Ion Arrays (MIAs)
Supramolecular chemistry, with its characteristic control of the self-assembly
process and its intrinsic defect tolerance, is a very efficient synthetic tool to
achieve ordered arrangements of metal ions with subnanometer precision [22]. In
order to study supramolecular entities under restricted spatial dimensions, in
particular on flat surfaces, scanning tunneling microscopy (STM) is the investiga-
tion method of choice due to its excellent real-space imaging and manipulation
capabilities. Recent advances in scanning probe techniques of molecules have
enabled imaging and also manipulation with partially sub-molecular resolution
[13c]. Thus, after bulk self-assembly synthesis, monolayers of [2
2] Co
4
II
metal
ion arrays can be generated simply by drop casting the molecules on an atomically
flat graphite surface (HOPG) (following way A in Fig. 12.3). The highly ordered







Search WWH ::

Custom Search