Information Technology Reference
In-Depth Information
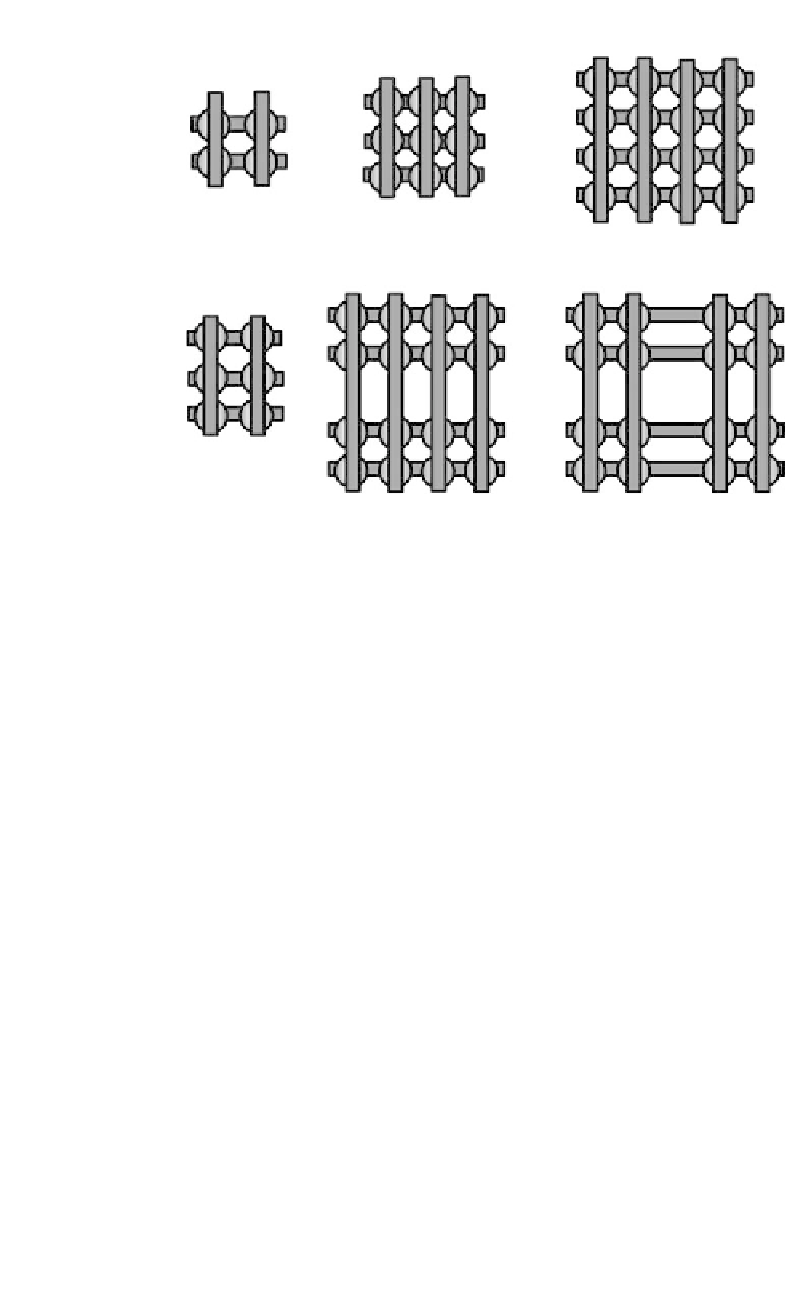
(a)
[2
×
2]
[3
×
3]
[4
×
4]
(b)
(c)
[2
×
3]
[2
×
[3
×
2]]
[4
×
[2
×
2]]
Figure
12.4.
Schematic representation of the different types of metal ion arrays
(MIAs): (a) [22], [33], and [44] squares, (b) [23] rectangles and (c) some
examples of more complex architectures of the [2 x [42]] and [4 x [22]] type
(reprinted from [10]).
enable bi- or even multistability at the nanometer scale. Among the promising
molecular parameters, the redox and the magnetic behavior of some [2
2] and
[3
3] metal ion arrays have been studied in detail.
In their bulk, some [2
2] Co
4
II
and [3
3] Mn
9
II
arrays have been proven to
be very efficient electron reservoirs exhibiting multiple and oxidation steps in
diluted solutions [14]. The [2
2] Co
4
II
metal ion array exhibits very extraordin-
ary reduction behavior in multiple (up to twelve), well resolved single electron
steps with wave separations between 20 to 40mV (Fig. 12.5a). Rather low redox
potentials (below 3 V) guarantee robust cycling for some Co
4
II
compounds; this is
a necessary prerequisite for low fatigue rates in future device stability. Note the
stability of the recuced species can be directly correlated to the nature of the
organic ligands and the metal ions, since replacing the Co
2+
ions by Fe
2+
,Zn
2+
,
Mn
2+
decreases the cyclability considerably [15]. In view of the realization of
molecular cellular automata (see Chapter 4), two basic results dealing with metal
ion arrays of the [2
2]Co family might be of interest: (i) The metal ion arrays can
be ordered along step edges of graphite into 1D infinite chains (Fig. 12.5b) [16]. (ii)
The step-wise synthesis of a [2
2]Co
I
2
Co
III
2
metal ion array yields molecular
species, which show exactly the diagonally localized electron density distribution
required for the internal cell structure of such automata devices (Fig. 12.5c) [17].




Search WWH ::

Custom Search