Environmental Engineering Reference
In-Depth Information
heat is extracted from the soil system. Ice starts to form at
0
◦
C. The temperature may be from -5 to -10
◦
C before all
the water in the soil is frozen.
The latent heat of fusion is often viewed as a disconti-
nuity that occurs as water freezes or thaws. However, in
reality, water in a soil gradually freezes as the temperature
is lowered. The reverse process occurs as ice thaws.
Heat out
Soil
particle
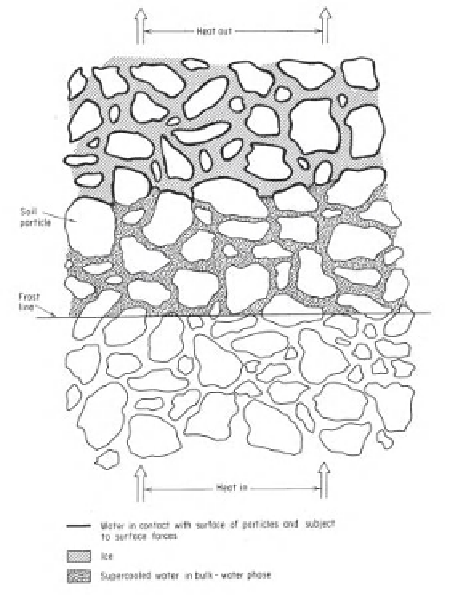
10.3.2 Freezing of Soils
The freezing of soils is usually driven by thermal conditions in
the atmosphere above the ground surface. A thermal gradient
develops between the cold atmosphere and the underlying
warmer soil (Fig. 10.5). A transient process is initiated that
involves conductive, convective, and radiation heat transfer.
However, the conductive heat transfer is generally dominant.
As the temperature of the atmosphere drops below the
freezing point, water in the pores of the soil begins to freeze
and releases latent energy into the system. The amount of
liquid water is reduced in the freezing zone and negative
pore-water pressures are generated in the freezing zone. The
thermal and suction gradients result in water flow toward
the freezing zone. The affinity of the ice-water interface for
more water can result in the buildup of ice lenses in the soil.
The frost front advances downward into the soil as long as
the frozen zone can remove the latent energy released by the
phase change. The suction gradients are high, calculated in
accordance with the Clapeyron equation. At the same time,
there is a reduction in the water permeability across the freez-
ing zone. The reduced water coefficient of permeability limits
the amount of water that can be delivered to the freezing front.
Harlan (1973) suggested that the frozen water reduces the
water coefficient of permeability in a similar manner to the
way that desaturation of a soil reduces the water coefficient
of permeability for an unsaturated soil. Partial freezing of the
soil gives rise to the soil suction versus water coefficient of
permeability relationship for the freezing soil.
Freezing
zone
0
°
C
Heat in
Water in contact with surface of particles and
subject to surface forces
Ice
Supercooled water in bulk-water phase
Figure 10.5
Idealization of partially frozen zone in soil (i.e., tran-
sition from water to ice in the voids).
proposed for describing the freezing behavior of fine-grained
soils (Harlan, 1973; Guymon and Luthin, 1974; Jame, 1977;
Konrad and Morgenstern, 1990; Nixon, 1992).
10.3.1 Latent Heat of Fusion
The volumetric latent heat of fusion
L
is the amount of
energy associated with phase change during the freezing or
thawing of a soil. The volumetric latent heat of water-ice
is 3
.
34
10
8
J/m
3
(Andersland and Anderson, 1978). The
latent heat consumption must be taken into consideration
when water is converted to ice and vice versa.
The latent heat of fusion applies only to the water phase
but can be written in terms of the total soil mass (Andersland
and Anderson, 1978):
×
10.3.3 Unfrozen Water Content
Boyoucous (1920) showed that part of the water in clay
remains unfrozen at temperature values as low as -78
◦
C.
Other researchers have attempted to explain the unfrozen
water content in terms of freezing point depression and the
freezing temperature as a function of the unfrozen water con-
tent (Williams, 1964, 1966; Nersesova and Tsytovich, 1966;
Miller, 1966). One of the most common forms for repre-
senting the freezing mechanism has been the relationship
of unfrozen water content to temperature below the freez-
ing point of pure water (Yong, 1965; Nersesova and Tsy-
tovich, 1966). Figure 10.6 shows temperature below freez-
ing versus water content for silt and clay soils compacted
at various water contents (Yong, 1965). Similar plots for
a variety of soils are shown in Fig. 10.7 (Nersesova and
Tsytovich, 1966). Jame (1972) showed that the relationship
between freezing point depression and water content was
3
.
34
w
ρ
d
ρ
w
10
8
L
=
×
(10.10)
where:
=
L
latent heat written in terms of the total soil sample,
J/m
3
,
w
=
gravimetric water content,
dry density, kg/m
3
, and
ρ
d
=
density of water, kg/m
3
.
ρ
w
=
The freezing of water in a soil does not occur instanta-
neously. Rather, some of the water is converted to ice as













Search WWH ::

Custom Search