Environmental Engineering Reference
In-Depth Information
V
=
total volume of soil,
0.68
10
6
ζ
w
=
heat capacity for the water phase (i.e., 4
.
2
×
0.66
J/m
3
/K for water at 20
◦
C),
θ
=
volumetric water content (i.e.,
V
w
/V
),
0.64
V
w
=
volume of water in the soil,
10
3
ζ
a
=
heat capacity for the air phase (i.e., 1
.
2
×
J/
0.62
m
3
/K), and
0.60
θ
a
=
volumetric air content (i.e.,
V
a
/V
).
0.58
Typical values for specific heat of water at various tem-
peratures are shown in Table 10.4.
It can be observed in Table 10.5 that volumetric heat is
equal to the heat capacity
C
w
multiplied by the density
ρ
w
of the material. For example, the volumetric heat capacity
of water can be written as follows:
0.56
0.54
0 0 0 0 0 0 0 0 0
Temperature,
°
C
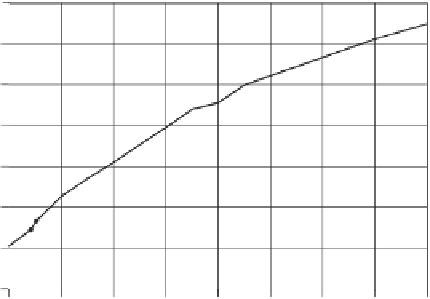
Figure 10.3
Thermal conductivity of water versus temperature.
ζ
w
=
ρ
w
C
w
(10.8)
10.2.4 Heat of Vaporization
The volumetric latent heat of vaporization or condensation,
L
,is2
.
5
10.2.3 Heat Capacity and Specific Heat
for Unsaturated Soils
Temperature change in a soil occurs in response to the adsorp-
tion or release of heat. The soil property describing the ability
of a material to adsorb or release heat is called volumetric
heat capacity. The heat capacity of a soil is dependent upon
the type of minerals present, the porosity of the soil, and the
relative portion of water and air in the voids (Baver, 1956).
Volumetric heat capacity is the term commonly used when
describing the heat storage capability of a soil-water-air mix-
ture. Specific heat is the term often used when referring to
each of the constituents of a multiphase system.
It is possible to calculate the volumetric heat capacity of
a multiphase system if the specific heat of each constituent
and the amount of each constituent is known. Considerable
attention has been given to the accurate measurement of the
specific heat of each constituent of a multiphase system. It is
also useful to know the specific heat of water as a function
of the various states in which it can exist (i.e., liquid, solid,
and vapor).
The heat capacity of a soil can be designated either with
reference to a unit volume or with respect to a unit mass.
Specific heat is commonly designated with respect to a unit
mass of material. Volumetric heat is referenced to a unit
volume. The volumetric heat capacity
ζ
of an unfrozen soil
consisting of solids, water, and air can be calculated using
the relationship given by de Vries (1963):
10
9
J/m
3
(Andersland and Anderson, 1978). The
latent heat of vaporization in terms of mass is 2
.
42
×
10
6
J/kg. The latent heat of vaporization can be applied during
a vaporization process which may occur when the tempera-
ture is greater than the freezing temperature. Condensation
is the reverse of vaporization and the latent heat term for
condensation is the same as for vaporization.
×
10.2.5 Diffusivity Constant
Thermal diffusivity is another term that is often used when
considering the analysis of heat flow. Thermal diffusivity
Table 10.4 Specific Heat Capacity
C
w
versus
Temperature for Water (Liquid Phase)
Temperature,
◦
C
Specific Heat Capacity
C
w
(J/g/K)
0
4.2161
4
4.2077
5
4.2035
10
4.1910
15
4.1868
20
4.1826
25
4.1784
30
4.1784
ζ
=
ζ
p
θ
p
+
ζ
w
θ
+
ζ
a
θ
a
(10.7)
35
4.1784
40
4.1784
45
4.1784
where:
50
4.1826
60
4.1843
ζ
p
=
heat capacity of the soil solids [i.e., a typical value
is 2
.
235
70
4.1895
10
6
J/m
3
/K for fine sands (de Vries,
×
80
4.1963
1963)],
θ
p
=
volumetric solid content (i.e.,
V
s
/V
),
V
s
=
volume of soils solids in the soil,
Source:
Data compiled from Lide (1992).



























Search WWH ::

Custom Search