Information Technology Reference
In-Depth Information
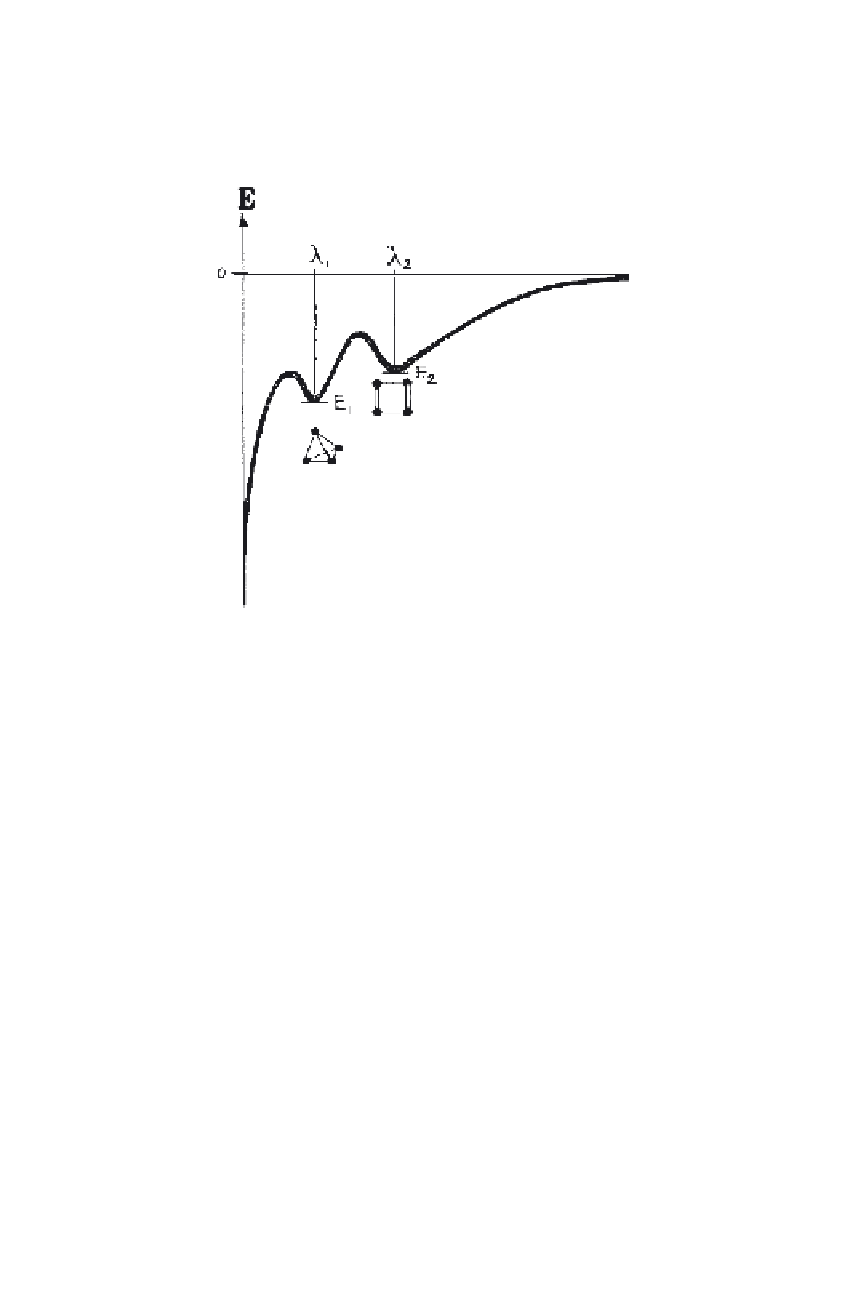
FIGURE 8. Association of energy levels with the two different configurations (iso-
meres) of a molecule composed of four atoms, each having three valences (n = 4;
V = 3). l
1
and l
2
represent the eigen-values in the solution of the Schrödinger wave
equation.
“forbidden” transitions.
28
Since being in such a state is the result of a par-
ticular energy transaction, selective “read-out” that triggers the transition
to the groundstate—as in the optical maser—permits retrieval of the infor-
mation stored in the excited states.
There is, however, another way to allow for information storage in macro-
molecules where the “read-out” is defined by structural matching (templet).
It is obvious that, m, the number of ways (isomeres) in which n atoms with
V valences can form a molecule Z
n
will increase with the number of atoms
that constitute this molecule. Each of these configurations is associated with
two characteristic energy levels (quantum states), one that gives the poten-
tial energy of this configuration, the other one which is the next higher level
at which this configuration becomes unstable. Figure 8 sketches this situa-
Search WWH ::

Custom Search