Biomedical Engineering Reference
In-Depth Information
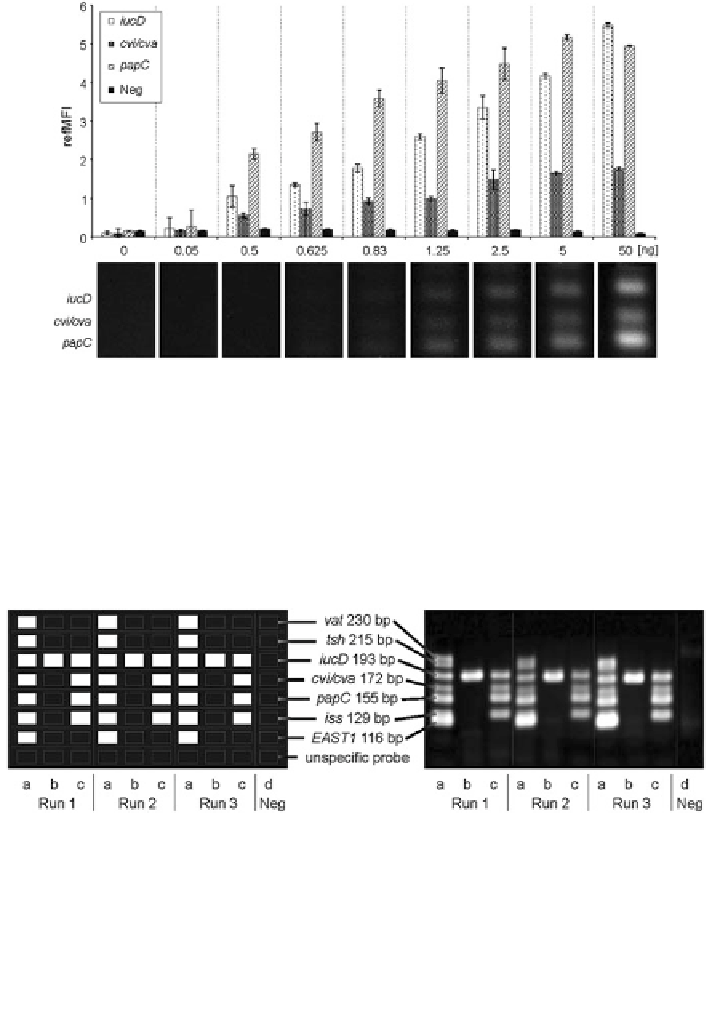
Fig. 14 Sensitivity/detection limit of the VideoScan hybridization assay: analysis of three VAGs
from E. coli (iucD, cvi/cva, papC) by the VideoScan hybridization assay in comparison to
standard agarose gel analysis. Comparison of refMFI and agarose gel analysis of a gradation of
template DNA amounts used for PCR (0-50 ng). Increasing refMFIs correspond to increasing
agarose gel band intensities. The signal pattern was independent of the PCR product quantity
used. Down to 0.83 ng PCR product, VideoScan and the agarose gel reported identical results.
However, below this, VideoScan was still able to detect PCR products. A gene non-specific
sequence served as negative control (Neg)
Fig. 15 Reproducibility of the VideoScan multiplex hybridization assay. Comparison of results
of three runs of simultaneous amplification of seven virulence-associated genes (names and
number of base pairs in the middle) of E. coli by VideoScan multiplex hybridization assay (left)
and standard agarose gel (right). Additionally, a non-specific probe was coupled to microbeads to
exclude non-specific hybridizations. Results for each run are similar between the VideoScan
multiplex hybridization assay and standard agarose gel analysis which is shown as an example for
the three E. coli strains IMT2470 (a), PS2506 (b), PS2507 (c). Positive values of refMFI which
are represented as white boxes (left), and bands in the agarose gel (right) correspond to the
detection of the gene of interest. d Negative control (Neg)
do not hybridize to the gene-specific capture probes. The VideoScan multiplex
hybridization assay is fast, sensitive and highly reproducible. On the basis of these
interesting features, we conclude that the VideoScan hybridization assay is a