Biomedical Engineering Reference
In-Depth Information
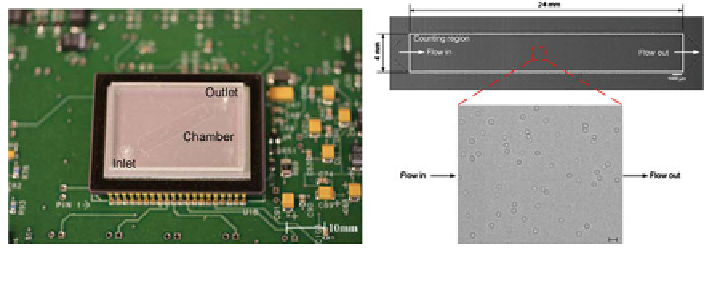
Fig. 12 Imaging CD4
+
T-lymphocytes on-chip using a lensless system. Reprinted from [
70
] with
permission from Elsevier
with the naked eye [
14
]. However, if the application demands higher sensitivity,
then alternative methods will need to be explored. An emerging topic that is
gathering more and more interest is optofluidics [
9
,
84
]. Here, microfluidic tech-
nology is combined with optics to realise highly compact and integrated devices.
The following will focus on the recent developments in integrated on-chip
detection methods that are suitable for molecular diagnostics.
Lensless systems. Monitoring the cell counts of patients in resource-limited
settings is currently performed using fluorescent activated cell sorting systems.
Although effective, these systems are expensive and require an experienced
operator [
118
]. The combination of optical technologies with microfluidic plat-
forms has led to the recent development of integrated lensless systems for POC
diagnostics [
39
]. The aim is to provide high resolution images of cells from a small
sample volume using a low-cost portable device. Applications of this emerging
technology include wide-field cell monitoring arrays [
91
-
93
,
103
], optofluidic
microscopy [
9
,
18
,
77
], and lensless on-chip microscopy [
76
,
107
].
A lensless system integrated with a microfluidic chip has been used to count
CD4
+
T-lymphocytes for monitoring HIV [
70
]. In this work, the anti-CD4 anti-
body was immobilised on the surface on an optically clear microchannel situated
above a CCD sensor (Fig.
12
). A 10 ll blood sample was diluted in serum, cen-
trifuged and the extracted serum was introduced into the chamber. The unbound
cells, such as red blood cells and unwanted CD4
+
monocytes, were then washed
away with a buffer solution. The remaining CD4
+
T-lymphocytes were imaged
using a lensless shadow imaging technique. White light from a guided light source
passes through the semi-transparent cells and casts a shadow on the sensor. The
light is partially scattered, creating a holographic shadow that can be processed
using the equivalent graphics-processing power found on a mobile phone. The
reconstructed images had sufficient resolution to distinguish the cells from the
background and allowed cell counting in 3 s. In nine separate devices, the results
showed an 88.8 ± 5% capture specificity for CD4
+
cells and a 70.2 ± 6.7%
capture efficiency. Moreover, when compared to the criterion standard of flow
cytometry, the overall performance was 83.5 ± 2.44%. The small 2.44% standard