Biomedical Engineering Reference
In-Depth Information
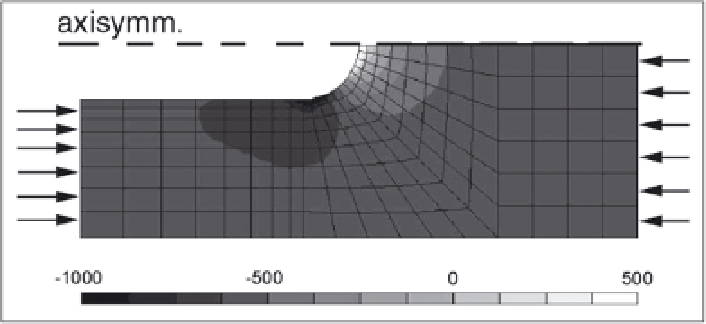
Figure 4. Volumetric strain within the bone matrix around the progressing end of an osteonic BMU at
maximum loading during during the walking cycle. The direction of loading is indicated by arrows. A
superficial area of volumetric expansion appears at the tip of the cutting cone. At the base of the (hemi-) cone
an area of high volumetric compression appears. Values are microstrains. Figure taken from Burger et al,
2002, with permission.
loading of the remodeling piece of bone. Decreased strain was found in front of the cutting
cone, just where resorption continues to proceed. Elevated strain however was found behind
the cutting cone, where osteoblasts are active.
103
This observation suggested that the subse-
quent activation of osteoclasts and osteoblasts in a BMU could be regulated by the local strains
in the surrounding bone. As these strains derive directly from the direction and magnitude of
the daily loads, we have an explanation how a BMU can produce a (hemi-) osteon that is
aligned to the prevailing mechanical loads of the piece of bone. In recent papers,
104,105
we
subsequently calculated the canalicular fluid flow in the bone around cutting- and closing cone
of a progressing BMU, using parameters for the poro-elasticity of cortical bone developed
earlier.
106
These studies show that physiological loading produces stasis of extra-cellular fluid in
front of the cutting cone of a progressing BMU, while enhanced extra-cellular fluid flow occurs
around the closing cone
105
(Figs. 4 and 5). Thus, osteoclastic resorption occurs in the direction
of nonstressed osteocytes, while osteoblastic bone formation occurs where osteocytes receive
enhanced fluid shear stress. Together these observations explain the alignment of the new
(hemi-)osteon along the direction of loading, as well as the refilling of the resorption tunnel or
trench by osteoblasts.
In vitro, fluid shear stress rapidly induces production of NO and prostaglandins (PG) by
osteocytes.
15,17
Both molecules are involved in maintaining the viability of the producing cell,
a form of autocrine regulation.
107
Lack of NO and PG production by cells has been related to
their programmed cell death or apoptosis.
107,108
We have therefore proposed that the produc-
tion of NO and PG by shear stressed osteocytes serves to keep them vital.
104
Absence of fluid
shear stress in front of the cutting cone will therefore lead to osteocyte apoptosis, as a result of
insufficient production of NO and PG. Apoptotic osteocytes have been shown to attract osteo-
clasts, which act as the macrophages of bone by phagocytosing the apoptotic osteocytes while
resorbing their extracellular matrix.
109-113
The progression of a BMU along the direction of
loading, and therefore the orientation of the new (hemi-)osteon that is formed, can thus be
explained. The magnitude of the daily loads however will determine the magnitude of the fluid
shear stress around the closing cone, which will determine the amount of activation of osteo-
blasts to refill the closing cone and thereby the amount of bone mass.
Mechanical Strain and DOG
Interfragmentary movement after bone fracture and distraction in DOG cause much larger
tissue strains (about 2% to 10%) then normal loading in bone does. These strains are largest in
Search WWH ::

Custom Search