Biomedical Engineering Reference
In-Depth Information
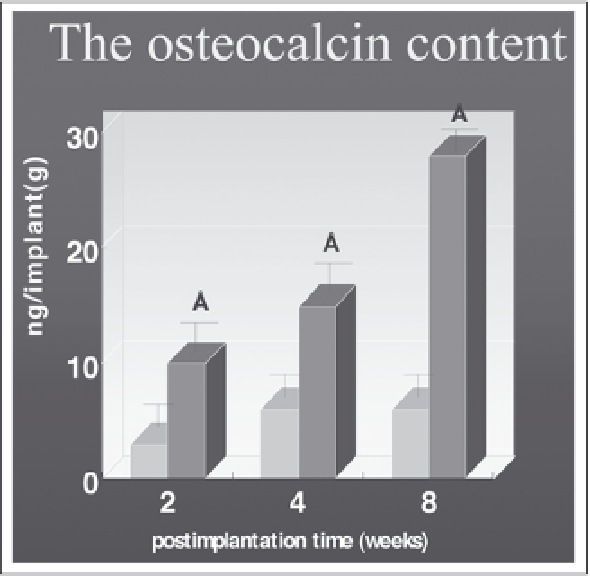
Figure 12. The osteocalcin content for mineral detection. The experimental samples (MSCs /fibrin glue-
β
-TCP
admixture) and control (fibrin glue-
β
-TCP admixture) harvested at 2, 4, and 8 weeks after injection.
Osteocalcin content increased over time in the tissue implants developed from the MSCs /fibrin glue-
β
-TCP
admixture. Each point represents the mean value of osteocalcin content
±
SE (n=5 at each point). Asterisks
indicate significant differences in osteocalcin at p<0.05.
areas were increased time-dependently. Despite of bone formation in vivo, no cartilage was
observed in the porous areas at any time.
Control implants with fibrin glue-
β
-TCP admixtures alone exhibited none of these histo-
logic features and did not show any bone formation in the area at 2, 4 and 8 weeks after
implantation and we observed only fibrous tissues. With time, the fibrin glue-
β
-TCP was
gradually resorbed, a result producing implants smaller and flatter and containing numerous
pores and fibrous tissues caused by the biodegradation of the fibrin-
β
-TCP admixture (Fig.
12). These changes were correlated with those found by x-ray and osteocalcin content for
mineral detection in the implants developed from MSCs/fibrin glue-
β
-TCP admixtures or
fibrin glue-
β
-TCP admixture.
Osteopontin, a protein important in bone development, was identified with experimental
groups, but it could not detect in the control groups. The osteocytes was positive with the
antibody. These results were consistent with the osteocalcin content, x-ray findings, and histo-
logic evaluations. In this regard, this study demonstrates that MSCs/
β
-TCP matrix composites
can be transferred with fibrin glue to recipient sites in animal models without loss or viability
of cultured tissue and fibrin glue allows MSCs proliferation without deforming cell structure
and is an appropriate delivery substance.
Discussion
In the context of minimally invasive surgery, the next logical step is to provide a biological
replacement for missing tissue without the need for a harvesting operation. Tissue engineering is
defined as the fabrication of living parts for the body from cells in the laboratory. Donor cells such
as stem cells or cultivated, differentiated cells are seeded on an appropriately configured scaffold
Search WWH ::

Custom Search