Biomedical Engineering Reference
In-Depth Information
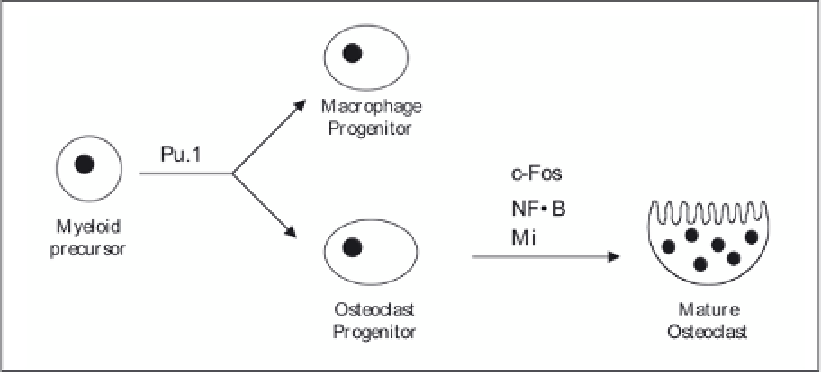
Figure 2. Transcription factors in osteoclast differentiation.
granulocytic cells.
40
Pu.1-deficient mice lack both osteoclasts and macrophages,
41
demonstrat-
ing that Pu.1 controls the differentiation of these two types of cells. The action of Pu.1 is
cell-autonomous since the defect was completely rescued by bone marrow transplantation
41
or
forced expression of Pu.1 in Pu.1-deficient monocyte progenitors.
42
Pu.1 -/- progenitors could
commit to the monocytic lineage but these cells could not mature to macrophages,
43
suggest-
ing that Pu.1 is only required at later stages of macrophage differentiation. Pu.1 is genetically
upstream of M-CSF in control of osteoclast differentiation (Fig. 2) as Pu.1 directs cell-specific
expression of M-CSF.
44
Early observation that FBJ murine osteosarcoma virus induces osteosarcomas in mice
45
had
drawn attention to v-Fos, the gene responsible for this malignant transformation. In situ hy-
bridization shows that c-Fos, the cellular counterpart of v-Fos, is expressed in cartilage, bone,
and tooth during mouse development
46
while overexpression of c-Fos in mice causes osteosar-
coma.
47
These results made c-Fos a good candidate to control osteoblast differentiation in vivo.
A major surprise came from later loss-of-function studies. Indeed, homozygous c-Fos -/- mice
develop osteopetrosis, a disease characterized by increased bone mass, due to the absence of
osteoclast,
48
demonstrating that c-Fos is required for the differentiation of monococyte precur-
sors into osteoclasts. The action of c-Fos is also cell-autonomous because the arrest of osteoclast
differentiation can be released by bone marrow transplantation or ectopic expression of c-Fos.
49
The fact that c-Fos-deficient mice also hav ane increased number of macrophages implicates a
reciprocal regulatory mechanism in which c-Fos may act as a repressor for macrophage matura-
tion. The absence of macrophages in c-Fos-deficient mice argues that c-Fos lies downstream of
Pu.1 in controlling osteoclast differentiation.
c-Fos shares high homology with Fra-1 at the DNA-binding (basic) and leucine zipper
domains but Fra-1 lacks a transcription activation domain.
50
Recently, mice carrying a Fra-1
allele inserted in the c-Fos locus were generated, these mice did not show any bone develop-
mental abnormality
51
suggesting the c-Fos-dependent osteoclast differentiation does not re-
quire the transactivation ability of c-Fos. This result is consistent with the observation that
Fra-1 overexpresion in a c-Fos-deficient mice rescues osteopetrosis phenotype.
52
However, Fra-1
is not required for osteoclast differentiation since Fra-1-deficient mice that have been rescued
by blastocyst injection contained functional osteoclasts.
36
Two forms of NF-
κ
B, P50 and P52 are closely related transcription factors regulating im-
mune and inflammatory responses. The transcription activity is tightly regulated
post-translationally.
53
Defects in animals caused by targeted deletion of either one of them are
restricted in immune systems. Knockout of the gene encoding for P50 leads to an impaired
ionizing radiation-induced NF-
κ
B activation,
54
whereas targeted mutation of P52 have
Search WWH ::

Custom Search