Biomedical Engineering Reference
In-Depth Information
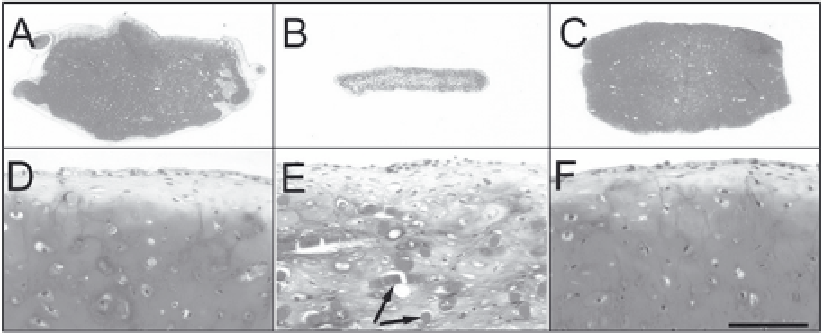
Figure 2. Cartilaginous tissues engineered using polyglycolic acid nonwoven meshes and bovine chondrocytes,
either freshly harvested (A, D) or expanded in monolayers without (B, E) or with (C, F) FGF-2. Medium
supplementation with FGF-2 not only increased chondrocyte proliferation rate, but also maintained in
chondrocytes a higher capacity to generate cartilaginous tissues, similar to that of freshly harvested cells.
77
We recently demonstrated that also human chondrocytes may be expanded while maintaining their ability
to redifferentiate, although in this case multiple growth factors are necessary.
78,79
Glycosaminoglycans are
stained red by Safranin-O. Arrows indicate undegraded polymer fibers after 4 week culture. Scale bar = 2
mm (A-C) or 0.2 mm (D-F).
Differentiated chondrocytes can also be obtained by enzymatic digestion of nonarticular,
hyaline cartilage tissues. For example, biopsies of nasal or rib cartilage can be removed under
local anaesthetic and by a less invasive procedure than removing tissue from specific areas of the
joint. Furthermore, since the donor site is not subjected to compressive forces, there is a mini-
mal risk of morbidity at the biopsy site. It was recently shown that as compared to articular
chondrocytes, human chondrocytes from the nasal septum proliferate approximately 4 times
faster and have a higher capacity to generate a cartilaginous tissue after monolayer expansion.
80
However, extensive data from in vivo experimental studies will be needed to demonstrate the
efficacy of nasal chondrocytes at articular sites.
Mesenchymal Progenitor Cells
An alternative to the use of differentiated chondrocytes is the use of cells with chondrogenic
differentiation capacity. Mesenchymal progenitor cells with a chondrogenic potential have been
isolated from a variety of tissues, including periosteum,
81
bone marrow
82
and synovial mem-
brane.
83
Potential advantages of using mesenchymal progenitor cells as compared to differenti-
ated chondrocytes include: (i) higher proliferative capacity, even from older individuals; (ii)
higher responsiveness to growth factors and signalling molecules; and (iii) ability to differenti-
ate into chondrocytes or osteoblasts according to the local environment. This latter possibility
would allow the use of the same cell graft to repair both the articular cartilage and the subchon-
dral bone.
There have been a few experimental studies on the repair of focal articular lesions using
mesenchymal progenitor cells from bone marrow
84-86
and from periosteal/perichondrial tis-
sues.
87,88
One of the earliest study
84
highlighted the possibility that the cartilage regenerated in
osteochondral defects by marrow-derived mesenchymal progenitor cells could get thinner and
fibrillated after prolonged time, consistent with the known characteristic of these cells to termi-
nally differentiate in vitro into hyperthrophic chondrocytes. In general, the data reported are
qualitative and no study has shown yet either consistent results or the generation of a func-
tional tissue comparable to native hyaline cartilage. In addition, no report has been published
so far regarding the clinical use of mesenchymal progenitor cells from any tissue source for
articular cartilage repair. Thus, to date these cells have been reported to remain an unrealised
promise for effective regeneration of articular cartilage lesions.
89
Search WWH ::

Custom Search