Biomedical Engineering Reference
In-Depth Information
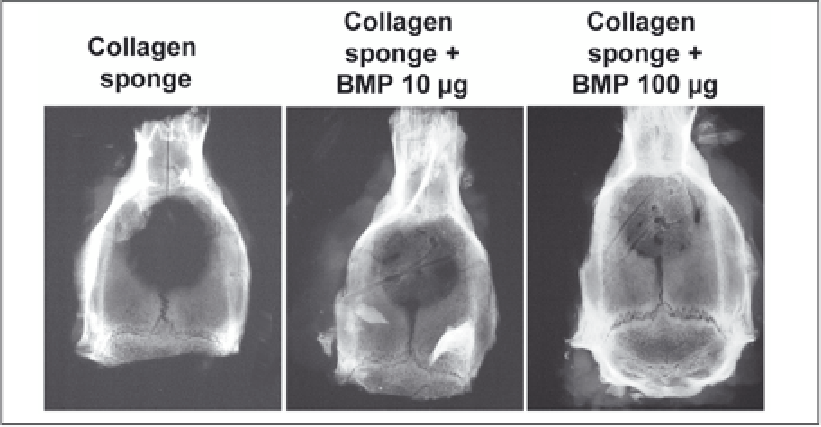
Figure 3. Radiographs of rat calvarial defects treated with collagen sponge with 0, 10 or 100
µ
g of BMP at
one month post-implantation.
process. It has been tested quite extensively with BMP (Fig. 3) in numerous animal models of
long bone healing,
9,84
maxillofacial reconstruction,
85-88
and spinal fusion.
12,89
Currently, it is
almost the only BMP delivery system used in clinical trials.
15,90,91
Friess et al looked at the interactions between rh-BMP-2 and absorbable collagen sponge
and the effects of the chemical and physicochemical properties of the carrier on the growth
factor binding.
92
From this study, it appears that rhBMP-2 binds to the collagen sponge effi-
ciently. The protein incorporation can be modulated depending on several parameters such as
material mass, soaking waiting time, liquid pH, and anion concentration.
As mentioned above, some investigators characterised the local pharmacokinetics of iodi-
nated BMP-2 released from collagenous carriers in the rat ectopic model. The results are clearly
presented in the review written by Winn et al.
93
As with most of the carriers, the pharmacoki-
netics of BMPs are biphasic. Some processes such as crosslinking with formaldehyde might
lead to a slight increase in in-vivo persistence, which may be related to slower degradation.
However, the type of sponge crosslinking process (chemical vs. dehydrothermal) as well as
ethylene oxide sterilisation do not affect the biphasic pharmacokinetics.
92
Gelatin is prepared through chemical denaturization of type I collagen. An interesting vari-
ant of gelatin hydrogels has been prepared through chemical crosslinking of acidic gelatin with
an isoelectrical point of 5. Sustained release of basic growth factors was obtained based on
polyion complexity.
94
Incorporation of rhBMP-2 or TGF
β
1 into acidic gelatin hydrogels en-
hance their bone regenerative potency significantly in a skull defect site in rabbits.
95,96
The use of collagen as a drug release system presents various advantages. It is available in
abundance, is biodegradable, hemostatic, and can be prepared in a number of different forms
including strips, sheets, sponges and beads. Functional groups of collagen side chains can be
chemically modified allowing the coupling of therapeutical agents such as antibiotics to
eventually improve the healing process.
97
However, biodegradation of collagen is a combina-
tion of a hydrolysis and an enzymatic process, which increases the difficulties in predicting
its in vivo behaviour. Standardisation of collagen extraction still remains a challenge. Addi-
tionally, an important disadvantage of collagen products consists of the constant risk of
pathogenic transmission.
Search WWH ::

Custom Search