Biomedical Engineering Reference
In-Depth Information
b
c
a
d
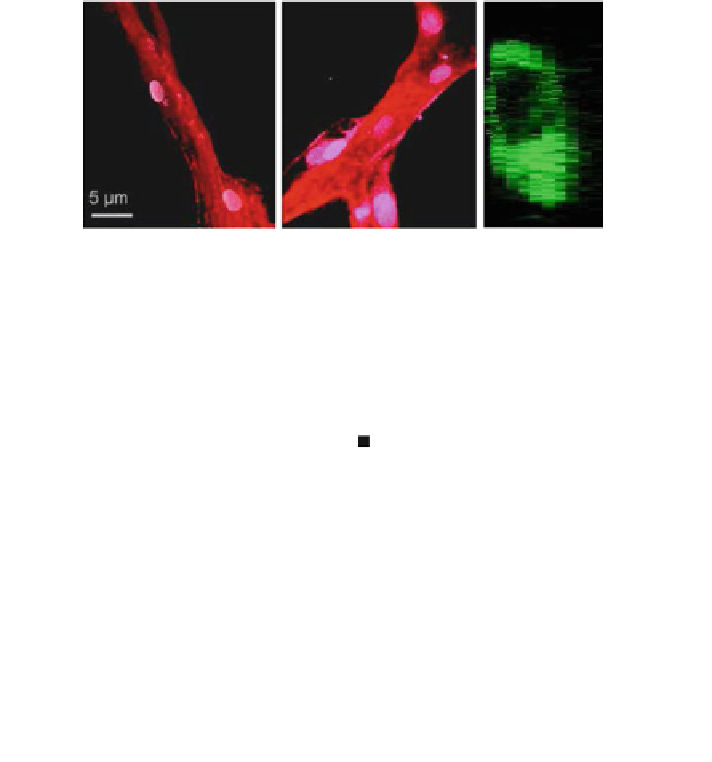
Fig. 9 Morphological markers of maturity. Representative confocal images of sprouts formed
within a 1.2 and b 1.9 mg/mL collagen matrices. Nuclei are stained white, actin cytoskeleton red.
c Confocal microscopy cross-sections of sprouts grown in 1.9 mg/mL collagen have a clearly
defined lumen. d Maturity is quantified in terms of lumen formation and cellular density. Extent
of lumen formation is the fraction of sprout length containing a lumen. Cellular density is
quantified as the number of cells per 10 microns of sprout length. Schematics of cross-sections
from each condition are shown. Error bars are standard deviation, n = 20. The average number of
cells per cross-section is two and three within 1.2 and 1.9 mg/ml matrices, respectively.
Originally published in [
69
]. Reproduced by permission of The Royal Society of Chemistry,
http://
the 1.2 mg/mL counterpart (Fig.
9
d). This faster rate of sprout maturation in the
higher density matrix could be due to increased mechanical resistance to elon-
gation, decreased proteolysis of the matrix, increased density of pro-proliferation
signaling from the matrix, decreased diffusivity of autocrine/paracrine secretions,
or some combination of these processes. Future studies utilizing well-defined,
tunable biomaterial matrices (as opposed to naturally harvested collagen), are
expected to shed light onto these mechanistic processes.
An additional micro-environmental cue that is thought to mediate sprout mat-
uration is interstitial flow. Recently, Kamm et al. have reported the development of
a microfluidic device that exposes the matrix directly to flowing reagent channels
in order to mimic the interstitial flow profile experienced in vivo [
71
]. Micropillars
inside the culture chamber stabilize the matrix to prevent degradation in response
to shear stress from the reagent streams. This device may be particularly beneficial






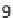




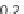











































Search WWH ::

Custom Search