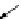Agriculture Reference
In-Depth Information
in harvesting machines that record the respective dry matter situation and simulta-
neously supply site-specific maps about this are state of the art.
Hence the logical procedure for defining the nutrient removal is:
•
harvested wet crop mass times dry matter content = harvested dry crop mass
•
harvested dry crop mass times removal per dry mass unit = nutrient removal
.
As for the units:
In Table
9.1
the nutrient removal is expressed per ton of dry matter of the har-
vested crop parts. A harvested wet crop mass per site-specific cell in t would have
to be multiplied by the dry matter content on the wet basis in decimal fractions and
not in %. The final nutrient removal would then be defined in kg of P
2
O
5
or K
2
O
per site-specific cell in the field.
9.2
Fertilizing Based on Soil Sensing by Ion-Selective
Electrodes
9.2.1
Basics
This method of detecting the site-specific nutrient supply of the soil relies upon the
electrochemical series of potential
(voltage) that holds when chemically different
conductors of electricity get into contact. As a result, the difference in potential can
cause a flow of electrons in case of metals or alternatively of ions in case of liquids
or slurries. The function of galvanic cells is based on this phenomenon.
However, with ion-selective electrodes the objective is not to produce electric-
ity, but just to use the indicated voltage for detecting specific ions. And in order to
get information about specific ions - such as those of the nutrients - the respective
electrodes contact the soil or the soil: water mixture via a
membrane
(Fig.
9.2
).
The function of this membrane is to transmit only the respective ions that are to be
sensed. So in case of soil sensing, membranes are selected that just transmit either
voltmeter
Fig. 9.2
Operating principle
of electrochemical sensing by
ion-selective electrodes. Both
electrodes can be within one
probe (simplified and not to
scale)
soil: water mixture