Geoscience Reference
In-Depth Information
Fig. 3.12
Ice
-
water interface
for freshwater ice and brackish/
saline ice. S refers to the
salinity of the water
S
~
1‰
S
~
1‰
0.1-1mm
dissolved substances out from the solid phase of water. However, due to constitutional
supercooling in the molecular diffusion of salt and heat in the liquid phase, cellular
ice
water interface forms (Fig.
3.12
) and is able to close liquid brine pockets between
crystal platelets (Weeks 1998). In fresh water, planar interface forms and separation is
much more effective.
The surface water salinity, where the transition between fresh water ice type and saline
ice type takes place, is within 1
-
according to observations in the Baltic Sea (Palosuo
1961; Weeks et al. 1990; Kawamura et al. 2001). Therefore brackish water ice is similar to
saline ice rather than to fresh water ice. The salinity of new ice is a fraction
2
‰
-
ʺ
of the
salinity of the water: Si
i
=
ʺ
S
w
, where
ʺ
is the segregation coef
cient,
ʺ ≈
0.25
-
0.5 for
ʺ
*
ä
brackish and saline ice (Weeks 1998) and
0.1 for freshwater ice (Lepp
ranta et al.
2003b). The segregation coef
cient increases with growth rate of ice. The salinity of liquid
brine within the ice must always correspond to the freezing point of the ambient tem-
perature, S
−
1
(T)=T
f
, and thus the brine salinity and consequently the brine volume
change with the temperature. In addition, in very low temperature salts start to crystallize
from the brine, each at its own eutectic temperature. The chemical composition of brine
and formation of salt crystals can be studied from the phase diagram of the lake water.
However, individual phase diagrams for particular freezing lakes are not known to the
author. Since seawater is a chemically uniform solution throughout the oceans, one phase
diagram is enough for all sea-ice (Assur 1958).
Brine volume is the primary factor to in
uence the properties of brackish and saline
ice, since brine inclusions may reach 10 % and more of the ice volume when the ice is
warm. Solid salt crystals scatter light and therefore in
fl
uence on the optical properties of
sea ice. Brine pockets serve as habitats of biota. They contain liquid water and nutrients,
and with light penetration, suf
fl
cient conditions for primary production exist. The algae are
captured into the ice in the freeze-up process, and their growth in the brine pockets is
primarily light-limited, in oligotrophic lakes likely also by nutrients. In saline lakes, the
most active layer is the bottom layer, so-called skeleton layer, which may become col-
oured brown-green by the algae.
In freshwater lakes, congelation ice does not provide an appropriate environment for
living organisms, but algae can grow in a slush layer between snow-ice and congelation
ice. The buoyancy of thinner ice is less, and therefore
flooding events are more common.
In very humic fresh water lakes, liquid humus layers have been found in the snow-ice
fl









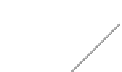




















Search WWH ::

Custom Search