Geoscience Reference
In-Depth Information
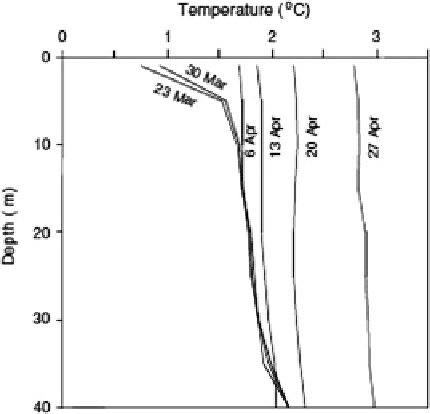
Fig. 7.8
Convective mixing in
Lake P
ää
j
ä
rvi, spring 2004
(Jakkila et al. 2009)
column becomes in a short time isothermal, apart from the very thin boundary layer just
beneath the ice to match the condition T = T
f
at the ice
water interface. Then, when the ice
cover breaks, due to mixing, melting can take place fast and right after disappearance of
ice the surface water temperature is well above zero.
The temperature effect on density is weak in the vicinity of the temperature of maxi-
mum density, and there is no (or, at most, weak) turbulence beneath a solid ice cover.
Therefore, even in freshwater lakes (S < 0.5
-
cation is affected by vertical
salinity gradients (Malm et al. 1997; Jonas et al. 2003). For a salinity difference of 0.1
‰
) the strati
,
the density difference is 0.08 kg m
−
3
, while in fresh water the density difference between
the temperatures of 0 and 4
‰
C is 0.13 kg m
−
3
. As a result, the in
es and
spreads in a thin layer just beneath the ice, if it has lower salinity than the lake water.
Apart from very deep zones, at the time of ice formation the temperature of bottom water
is normally below the temperature of maximum density, T
b
< T
m
. Therefore the heat
°
fl
ow water strati
fl
ux
from sediments creates denser water, which
fl
ows down toward greater depths.
Assuming that the ice is salt-free, we have
H
H
h
S
0
S
1
¼
ð
7
:
20
Þ
where S
0
and S
1
are the salinities before and after ice formation, respectively, H is the
depth of the convective layer, and h is ice thickness. In the growth of ice, taking H <1m
(Granin et al. 1999), for h = 0.1 m we have S
1
> 1.1
0.8 kg m
−
3
.
·
S
0
, i.e.
ʔρ
*
S
0
[
‰
]
×
Thus the higher the initial salinity is, the stronger the destabilizing in
uence will be.
Melting gives an opposite, stabilizing effect, and therefore the depth H comes from
molecular conduction and is much smaller than in the case of ice growth.
fl


Search WWH ::

Custom Search