Biomedical Engineering Reference
In-Depth Information
Development team
Average
Proj. Coord.
Systems Eng.
Proj. Man.
Comp. Sci.
Elect. Eng.
Doctor
Nurse
Administrator
Interface/
management
Users
Marketing/
communication
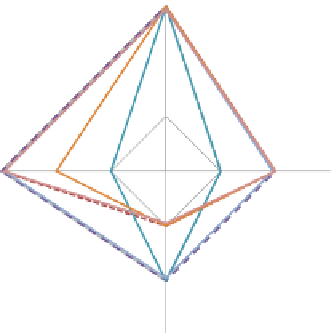
Fig. 5
Assessment of the degree of challenges, in a 1 to 4 scale, facing each of the 4 key groups
typically involved in the development of medical devices. Each line corresponds to one inquired
individual. The diamond symbols represent the average value for each group
a healthcare provider, who pointed out that investors and insurance companies are
vital parts of the creation of new medical devices, although the latter only become
key actors at the productification stage.
Subsequently, the questionnaire included three questions, each to be answered in
a matrix with multiple options. These questions pertained to:
•
Which of the groups (development team, medical staff, marketing, and coordina-
tors/managers) have to deal with the most critical challenges in the process?
•
Who has to become convinced by the developed solution?
•
At which point of the development process (early or late stage) should each actor
become involved?
The answers to each of these three questions were analyzed and the respective
results are shown in the following section.
5.2
Survey Results
The first question aimed at identifying the level of challenges that each of the
the four identified groups (development team, medical staff, marketing, and coor-
dinators/managers) have to deal with the most critical challenges in the process.
In the survey, a rating between 1 and 4 had to be assigned to each of these
groups. The results of the survey for different individuals, covering a range
of professional profiles, are shown in Fig.
5
. These profiles were, respectively,
the global project coordinator, a systems engineer, a project scientific manager,