Graphics Programs Reference
In-Depth Information
20.
P
T
P
2
2
Isothermal
expansion
Heating at
constant volume
P
T
1
T
2
Volume reduced
by cooling
1
V
V
V
2
1
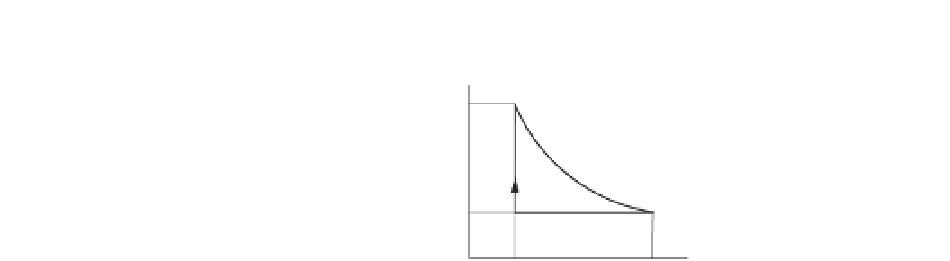
The figure shows the thermodynamiccycle of an engine. The efficiency of this
engineformonatomic gas is
ln(
T
2
/
T
1
)
−
(1
−
T
1
/
T
2
)
η
=
ln(
T
2
/
T
1
)
+
(1
−
T
1
/
T
2
)
/
(
γ
−
1)
where
T
is the absolute temperature and
γ
=
5
/
3. Find
T
2
/
T
1
that results in 30%
3).
21.
Gibb'sfree energy of one mole of hydrogenattemperature
T
is
efficiency (
η
=
0
.
RT
ln
(
T
T
0
)
5
/
2
J
G
=−
/
where
R
=
8
.
31441 J/K is the gasconstant and
T
0
=
4
.
444 18 K.Determine the
10
5
J.
temperature at which
G
=−
22.
The chemicalequilibrium equationinthe production of methanol fromCO
and H
2
is
8
)
2
ξ
(3
−
2
ξ
=
249
.
2
−
ξ
)
3
(1
.
23.
Determine the coordinates of the two points where the circles (
x
where
ξ
is the
equilibrium extent of the reaction
.Determine
ξ
2)
2
y
2
−
+
=
4
and
x
2
3)
2
4 intersect.Start by estimating the locations of the points from
a sketch of the circles, and thenuse the Newton-Raphsonmethod to compute
the coordinates.
24.
The equations
+
(
y
−
=
sin
x
+
3 cos
x
−
2
=
0
cos
x
−
sin
y
+
0
.
2
=
0
have a solutioninthe vicinity of the point(1
,
1)
.
Use the Newton-Raphsonmethod
to refine the solution.
8
FromAlberty, R.A.,
Physical Chemistry
, 7th ed., Wiley, 1987.



Search WWH ::

Custom Search