Environmental Engineering Reference
In-Depth Information
moval rates were shown. Most of the TBT was lost from the water column
through biodegradation which occurred at a rate of 0.08 day
-1
.Two-thirdsof
the degradation proceeded through debutylation to DBT which in turn de-
graded to MBT at
0.04 day
-1
. One-third of the TBT was degraded directly
to MBT in the water. Another portion of the TBT removed from the wa-
ter column was apparently transported rapidly to the air-water interface to
a measurable degree, and then lost from the tank as shown in Fig. 4 [98].
De Mora et al. [85] reported on sediment cores collected from Tamaki Es-
tuary in Auckland, New Zealand in 1989. The data from three stations were
used for the determination of a degradation rate for TBT in the sediment. The
core profiles from these stations display a high degree of internal consistency
when corrections are made for the differing sedimentation rates at each of
the stations. A plot of the natural logarithm of concentrations against time
yielded a straight line in all cases, thereby indicating that the degradation was
a first order kinetic process. The average degradation rate was 37.5%
∼
/
year
giving a half-life of 1.85 year [85].
4
Bioaccumulation and Sediment Accumulation of Organotin Antifoulants
Many studies have shown that TBT is not persistent in most natural wa-
ters. However, the tendency of TBT to accumulate in sediment and biota
implies that degradation processes operating in the sediment and biota will
be of greater importance in determining the overall persistence of TBT in the
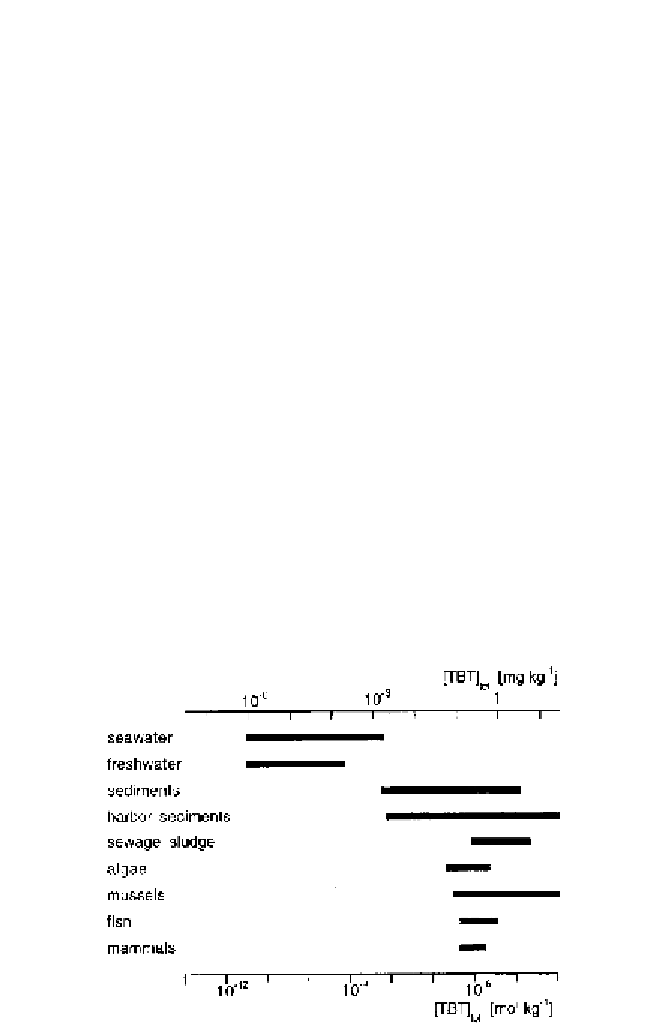
Fig. 5
Range of measured TBT concentration in different compartments of aquatic envi-
ronments [99]