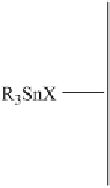Environmental Engineering Reference
In-Depth Information
Fig. 3
Environmental degradation scheme for TBT and TPT compounds [41]
The degradation of the organotin compounds is caused by hydrolysis,
ultraviolet light and microorganisms. The most dominant degradation is
caused by microorganisms such as bacteria, algae and fungi [24].
Almost all of the sun's emitted radiation in the UV region with wavelengths
shorter than 290 nm is absorbed by a thin band of ozone. However, the light
of 290 nm wavelength possesses an energy of approximately 300 kJ mol
-1
,and
this is above the typical range [42] of tin-carbon bond dissociation energies
(190-200 kJ
mol). Therefore, if absorption of light by the organotin takes
place, degradation can occur [24].
Mailhot et al. [43] pointed out that photodegradation by a photoredox pro-
cess by iron(III) occurs. Upon irradiation at
/
excitation
>
300 nm, a photoredox
process yielding iron(II) and OH radicals was observed. The disappearance of
TBTwasprovedtoinvolveonlyanattackbyOHradicalsinthepresenceof
iron(III): the quantum yield of TBT disappearance was determined.
λ
Fe
2+
+
·
OH + H
+
Fe
3+
+H
2
O
→
(3)