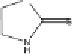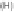Environmental Engineering Reference
In-Depth Information
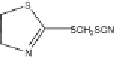
Fig. 10
Photolytic degradation pathways of TCMTB and MTB in aqueous environment,
based on reported identified phototransformation products [93, 96]
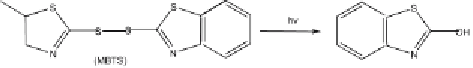
formation of the obtained photoproducts according to Brownlee et al. [93] is
depicted in Fig. 11.
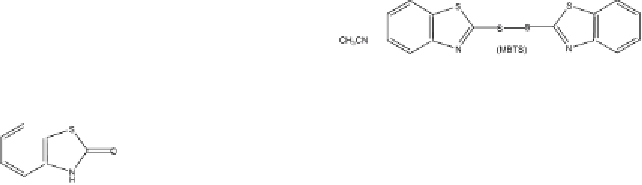
Thus, upon sunlight irradiation, MBT cleaves at the sulfur-hydrogen bond.
Subsequent recombination of the thiyl radicals formed leads to the disulfide
MBTS (a) [99]. These intermediates have been proposed not only by Ab-
dou et al. [98] but also by Párkányi and Abdelhamid [97]. Irradiation of the
MBTS, besides the retro S-S cleavage into to two thiyl radicals (b) [99], an-
other homolysis can be discussed leading to disulfan radical (c) as well as
to 2-benzothizolyl radical (d). This type of competitive carbon-sulfur cleav-
age has been investigated earlier [100] for the photoreactions of disulfide.
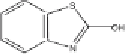
Radical (d) leads to benzothiazole BT (e) through a hydrogen abstraction
mechanism, while 2(3H)-benzothiazolone (OBT) (h) is formed by oxygen
Fig. 11
Photolytic degradation pathways and main transformation products of MTB in
aqueous environment (after Brownlee et al. [93])