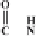Environmental Engineering Reference
In-Depth Information
Fig. 5
Photolytic degradation pathways and main phototransformation products of Sea-
Nine 211 in aqueous environment [34, 60]
this route also accounts for other processes such as hydrolysis and biodegra-
dation [39, 61]. In the case of distilled water, degradation owing to hydrolysis
(dark experiment) accounted for 30% of the decline in Sea-Nine 211 concen-
tration. In lake water, dark controls analyses (hydrolysis and biodegradation)
have shown a 65% contribution to the transformation of the biocide, indicat-
ing that biodegradation is considerable [55]. Further oxidation of
N
-
n
-octyl
acetamide leads to the formation of
N
-
n
-octyl oxamic acid, which could be
then phototransformed to the corresponding
N
-
n
-octyl carbamic acid as in-
dicated by Thomas [39] in the metabolic pathway of Sea-Nine 211 under
aerobic conditions. The presence of
n
-octyl isocyanate identified by Sakkas
et al. [60] may support the formation of
N
-
n
-octyl carbamic acid since it
reacts rapidly with water in a nucleophilic addition step generating the cor-
responding
N
-
n
-octyl carbamic acid. Although unstable in water its presence
may be attributed to the loss of water of
N
-
n
-octyl carbamic acid, probably
due to a thermal process prior to ionization in the GC-MS chamber.
N
-
n
-
Octyl carbamic acid decarboxylates yielding
n
-octyl amine.
The second pathway (b) consists on the phototransposition of Sea-
Nine 211. Photochemical interconversion of 1,2 into 1,3 isomers has been
reported for dihetero compounds, e.g., isoxazole to oxazole, imidazole to
pyrazole, as well as isothiazolone to thiazolones [62-64]. Photoexcitation of
isothiazolones is suggested to result in cleavage of the N - Sbond,result-