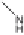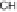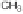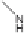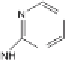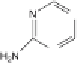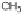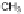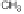Environmental Engineering Reference
In-Depth Information
Two routes of degradation pathways are observed during the photodegrada-
tion of irgarol 1051 according to the proposed reaction scheme (Fig. 4). The first
route involves the oxidation of irgarol's sulfur atom leading to the formation
of sulfone (compound 5). The cleavage of the sulfur group of the triazine ring
results in the formation of 2-hydroxy-4-
tert
-butylamino-6-cyclopropylamino-
s
-triazine (compound 2). This observation is in agreement with other studies
describing the direct photolysis of
s
-triazines where photoreaction must not
involve the alkyl group but the methylthio or the chlorine group, support-
ing the idea that hydroxy derivative formation is a major pathway in direct
photolysis [49, 50]. The same observation has been also reported during the
photocatalytic degradation of irgarol 1051 [51]. Thus, the formation of the
mono-dealkylated derivative, i.e., 2-methylthio-4-
tert
-butylamino-6-amino-
s
-
triazine (GS26575), was greatly favored over the hydroxylated and the sulfonyl
derivative since it occurred for more than 90%ofphotoreactions(according
to relative abundance of the compounds) and was attributed to indirect pro-
cesses. Okamura et al. [42] have also reported the presence of this byproduct as
the main one during irgarol 1051 photolysis in distilled water, while Torrents
et al. [52] have observed the formation of chlorodealkylated derivatives in the
case of atrazine during direct photolysis. Irradiation of aqueous irgarol 1051
solutions containing dissolved organic matter during simulated solar irradi-
ation, resulted in the formation of diaminohydroxy-
s
-triazine (compound 1),
Fig. 4
Photolytic degradation pathways and main phototransformation products of ir-
garol 1051 in aqueous environment [34, 47]