Environmental Engineering Reference
In-Depth Information
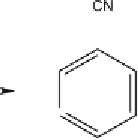
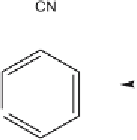
Fig. 2
Photolytic degradation pathways and main phototransformation products of
chlorothalonil in aqueous environment [31, 34]
The distribution pattern and the total number of degradation products dif-
fered between the various water samples and were dependent on the constitu-
tion of the irradiated media. In general the number of degradation products
was greater in natural and humic water than in distilled water. However, the
presence of common transformation products (compounds 2, 3, and 4) in dis-
tilled and in natural waters indicate that chlorothalonil is susceptible to both
direct and indirect photolytic reactions in natural waters.
3.2
Dichlofluanid
The photodegradation half-life in natural seawater was 53 h [35] and a half-
life of 18 h was reported using a bioassay method [36].
The photodegradation rate of dichlofluanid (
N
-dichlorofluoromethylthio-
N
,
N
-dimethyl-
N
-phenylsulfamide) was lower in natural waters than in
distilled water following the order: lake water
<
river water
<
sea water
<
distilled water, showing a strong dependence on the constitution of the
irradiated media and especially on the concentration of dissolved organic
matter [35]. As the TOC concentration increases in natural waters the rate of
photolysis decreases.