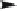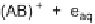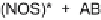Environmental Engineering Reference
In-Depth Information
2
Photochemical Transformation Processes
of Pollutants in Aqueous Environment
The appearance of trace amounts of micropollutants that occur in sur-
face water and groundwater has caused an increasing public and scientific
concern. Their fate in the aqueous environment is often unknown, how-
ever, direct and indirect photochemical processes may contribute to the
phototransformation
photodecomposition of these compounds in natural
waters. Mostly these reactions can occur simultaneously in natural waters,
therefore it is essential to consider both processes when examining the pho-
tochemical behavior of micropollutants. Besides the degradation kinetics,
literature reports on the photodegradation products of micropollutants is
relatively abundant [12]. However, little information is available on the re-
action mechanisms involved in the photolysis under typical environmen-
tal conditions. For environmental considerations it is important that they
can eventually be converted to innocuous, and preferably mineral, photo-
products.
/
2.1
Direct Photolysis
Most biocides absorb light at relatively short UV wavelengths. Since sunlight
reaching the Earth's surface (mainly UV-A, with varying amounts of UV-B)
contains only a very small amount of short wavelength UV radiation [13, 14],
the direct photodegradation of biocides by sunlight is expected to be, in most
Fig. 1
Direct and photosensitized transformation processes of micropollutants in the
aqueous environment