Environmental Engineering Reference
In-Depth Information
1
Introduction
As described in other chapters in this topic the chemical fate of antifoulants
is determined by many complex and interacting physical, chemical, and bio-
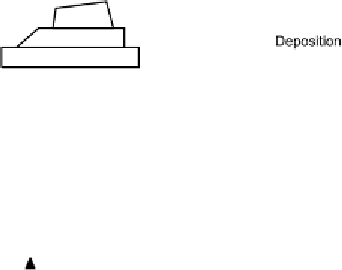
logical processes. Some of the major transport and transformation processes,
which are commonly considered in environmental fate models, have been sum-
marized in Fig. 1. The relative importance of each of the processes and pathways
is highly compound- and habitat specific and may have a considerable spa-
tial and temporal variation. For compounds with a high affinity to particulate
matter or sediment, sediment transport phenomena will be of dominant im-
portance. Stable dissolved compounds are likely to be affected most by river
discharges or tidal currents. Biodegradation processes are highly temperature
dependent and may be the dominant removal process in tropical water, while
in temperate or polar zones this may be less. Photolysis may have a prominent
role in the open sea even at greater depths in warm and transparent waters,
while in turbid estuarine environments in temperate zones this may only be
of importance in the upper water layers. Proper and realistic handling of pho-
tolysisinchemicalfatemodelsiscomplicated,asitvarieswithdepth.Thiscan
only be done in adequate 3D models with proper relationships of effective rate
constants with light penetration (transmittance or turbidity), temperature, and
binding to particulate matter and DOC.
There is a broad distinction between organic and inorganic compounds
both in the mechanisms and relative importance of different processes. For
Fig. 1
Chemical fate processes of antifouling products in the marine environment.
Source:[5].Reproducedwithpermission