Agriculture Reference
In-Depth Information
Heat
Respiration
Heat
Heat
Producers
Respiration
Herbivores
Carnivores
Top carnivores
Decomposition
and waste
Decomposer
biomass and heat
Net primary
productivity
Decomposer
biomass and heat
Decomposition
and wasted
food
Decomposer
biomass and heat
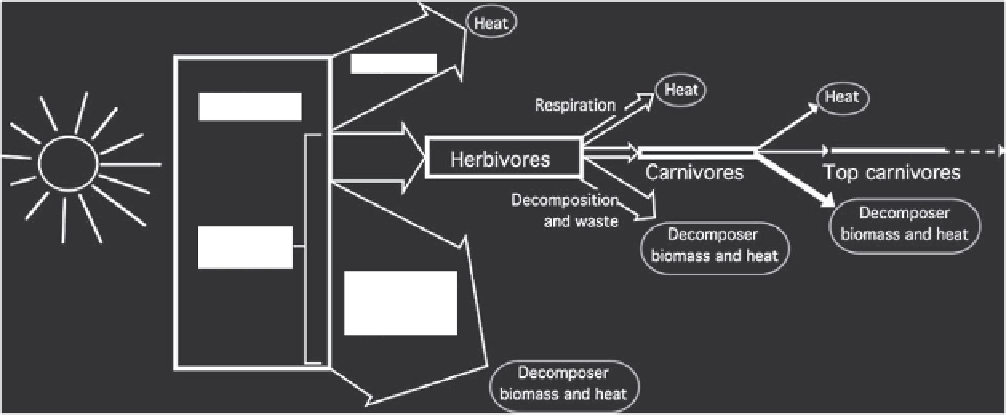
FIGURE 2.2
Ecosystem energy flow. The size of each box represents the relative amount of energy flowing through that trophic
level.
In the average ecosystem, only about 10% of the energy in a trophic level is transferred to the next trophic level. Nearly all the
energy that enters an ecosystem is eventually dissipated as heat.
between trophic levels contains both energy in chemical
bonds and matter serving as nutrients. Energy, however,
flows in one direction only through ecosystems — from
the sun to producers to consumers to the environment.
Nutrients, in contrast, move in cycles — through the biotic
components of an ecosystem to the abiotic components,
and back again to the biotic. Since both abiotic and biotic
components of the ecosystem are involved in these cycles,
they are referred to as
biogeochemical cycles
. As a whole,
biogeochemical cycles are complex and interconnected; in
addition, many occur at a global level that transcends
individual ecosystems.
Many nutrients are cycled through ecosystems. The
most important are carbon (C), nitrogen (N), oxygen (O),
phosphorus (P), sulfur (S), and water. With the exception
of water, each of these is known as a
macronutrient
. Each
nutrient has a specific route through the ecosystem
depending on the type of element and the trophic structure
of the ecosystem, but two main types of biogeochemical
cycles are generally recognized. For carbon, oxygen, and
nitrogen, the atmosphere functions as the primary abiotic
reservoir, so we can visualize cycles that take on a global
character. As an example, a molecule of carbon dioxide
respired into the air by an organism in one location can
be taken up by a plant halfway around the planet. Elements
that are less mobile, such as phosphorus, sulfur, potas-
sium, calcium, and most of the trace elements, cycle more
locally, and the soil is their main abiotic reservoir. These
nutrients are taken up by plant roots, stored for a period
of time in biomass, and eventually returned to the soil
within the same ecosystem by decomposers.
Some nutrients can exist in forms that are readily
available to organisms. Carbon is a good example of such
a material, easily moving between its abiotic form in the
atmospheric reservoir to a biotic form in plant or animal
matter as it cycles between the atmosphere as carbon
dioxide and biomass as complex carbohydrates. Carbon
spends varying lengths of time in living or dead organic
matter, or even humus in the soil, but it returns to the
atmospheric reservoir as carbon dioxide before it is
recycled again. Figure 2.3 is a simplified depiction of
the carbon cycle, focusing on terrestrial systems and
leaving out the reservoir of carbon found in carbonate
rocks.
Nutrients in the atmospheric reservoir can exist in
forms much less readily available and must be converted
to some other form before they can be used. A good
example is atmospheric nitrogen (N
2
). The conversion of
molecular nitrogen (N
2
) to ammonia (NH
3
) through bio-
logical fixation by microorganisms begins the process that
makes nitrogen available to plants. Once incorporated into
plant biomass, this “fixed” nitrogen can then become part
of the soil reservoir and eventually be taken up again by
plant roots as nitrate (NO
3
). As long as this soil-cycled
nitrogen is not reconverted back to gaseous N
2
or lost as
volatile ammonia or gaseous oxides of nitrogen, it can be
actively cycled within the ecosystem (Figure 2.4). The
agroecological significance of the biotic interactions
involved in this cycle are discussed in more detail in
Chapter 16.
Phosphorus, on the other hand, has no significant gas-
eous form. It is slowly added to the soil by the weathering
of rock, and once there, can be taken up by plants as
phosphate and then form part of the standing crop, or be
returned to the soil by excretion or decomposition. This
cycling between organisms and soil tends to be very local-
ized in ecosystems, with two major exceptions: (1) phos-
phates may leach out of ecosystems in ground water if they







Search WWH ::

Custom Search