Biomedical Engineering Reference
In-Depth Information
Vessel
pruning
Haemo-
dynamics
New
vessels
Radius
Haematocrit
Oxygen
VEGF
Normal cells
Cancer cells
- Cell-cycle proteins
- p53
- VEGF
- Cell-cycle proteins
- p53
- VEGF
Endothelial
sprouts
Fig. 3.1
Multiscale model overview (interaction diagram). This figure shows the connections
between the different modelling layers. In the subcellular layer, the cell cycle protein
concentrations and the p53 and VEGF concentrations are modelled via systems of coupled
ordinary differential equations. The local external oxygen concentration influences the duration
of the cell cycles. Cells consume oxygen, and produce VEGF in the case of hypoxia. Extracellular
VEGF also influences the emergence of endothelial sprouts and their biased random walk towards
hypoxic regions. If endothelial sprouts connect to other sprouts or the existing vascular network,
new vessels form. Vessel diameter is influenced by the local oxygen concentration and flow-
related parameters, such as pressure and wall shear stress. The vascular network delivers oxygen
throughout the tissue
3.2 Multiscale Model
The computational model that we use describes the spatio-temporal dynamics of a
tumour located in a vascular host tissue. Cells are represented as individual entities
(agent-based approach), each with their own cell cycle and subcellular-signalling
machinery. Nutrients are supplied by a dynamic vascular network, which is subject
to remodelling and angiogenesis. Interactions between the different layers are
depicted in Fig.
3.1
.
Our model is formulated on a regular grid that subdivides the simulation domain
into lattice sites. Each lattice site can be occupied by several biological cells whose
movement on the lattice is governed by reinforced random walks, and whose
proliferation is controlled by a subcellular cell cycle model. The vascular network
consists of vessel segments connecting adjacent nodes on the lattice, with defined




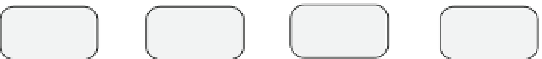













Search WWH ::

Custom Search