Biomedical Engineering Reference
In-Depth Information
a
b
Contrast berfore
administration
Contrast after
administration
Contrast before administration
9L GFP
9L-RFP
9L GFP
9L-RFP
100
P -
0
.
0012
P -
0
.
0049
90
80
70
60
50
40
30
20
10
0
NS
9L GFP
9L-RFP
Contrast after administration
9L GFP
9L-RFP
9L-GFP
9L-RFP
Muscle
c
White light
NIRF
Color coded NIRF
9L GFP
9L-RFP
d
White light
NIRF
Color coded NIRF
Muscle
9L-RFP
9L-GFP
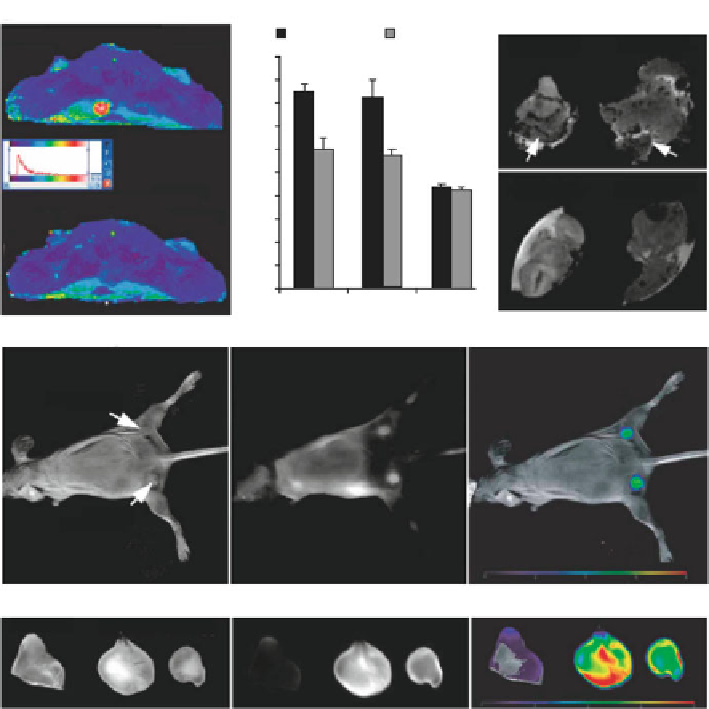
Fig. 1.4
(
a
) In vivo MR imaging of mice bearing bilateral 9L-GFP and 9L-RFP tumors before and
24 h after MN-NIRF-siGFP administration. A significant drop in T2 relaxivity was observed in the
tumors. (
b
) Ex vivo high-resolution MR images of excised tumors. (
c
) In vivo NIRF optical
imaging of tumor-bearing mice. The fluorescence signal associated with the tumors confirmed the
delivery of the MN-NIRF-siGFP probe to tumor tissues. (
d
) Ex vivo NIRF optical imaging showed
a significantly higher fluorescence in tumors than in muscle tissues (reprinted from [
21
] with
permission from Nature Publishing Group)
heat-generating nanoparticles undergo cell death when the temperature exceeds
their physiological tolerance. This event was first investigated in 1957 by Gilchrist
et al. where an external magnetic field was used to heat up tissues containing
magnetic nanoparticles [
8
]. Considering the fact that tumor cells are more sensitive
to temperature and that they uptake the magnetic nanoparticles more readily com-
pared to nonmalignant cells, a directed thermal change via magnetic nanoparticles is
a promising way to eradicate cancerous cells.
There have been numerous advances in the field of MFH. In vivo studies have been
performed in mice, rats, rabbits and dogs and for different types of cancers, including
Search WWH ::

Custom Search